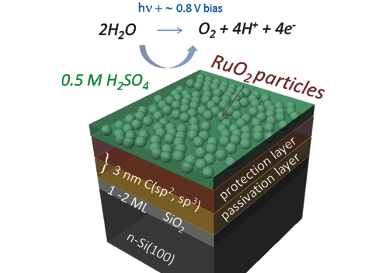
The artificial production of hydrogen from sunlight and water by semiconducting absorbers is a promising approach to generate environmentally friendly energy. State-of-the-art semiconductors have a high photovoltage and photon-to-charge-carrier conversion efficiency, but still lack of durability due to their tendency to degradation and passivation in acidic electrolytes.
Anahita Azarpira, Helmholtz-Zentrum for Materials and Energy GmbH, Berlin, Germany, and colleagues addressed this problem by the addition of an ultrathin organic protection layer in a two-step fabrication scheme. Firstly, hydrogen-terminated n-type silicon electrodes were exposed to an ethanol/iodine electrolyte. Under cathodic conditions the ethanol is activated by the iodine and subsequently reacts with the H-terminated silicon surface. In a second step, partly hydrated RuO2 powder catalyses the polymerization of ethanol resulting in a protection layer formed by polymerized ethyl-groups. RuO2 then proceeds to its original task in silicon electrodes: to catalyze the splitting of water to hydrogen and oxygen.
The resulting mulit-layer photoanode shows high stability and surpasses existing benchmarks of the photovoltage.
- Sustained Water Oxidation by Direct Electrosynthesis of Ultrathin Organic Protection Films on Silicon,
Anahita Azarpira, Thomas Schedel-Niedrig, H.-J. Lewerenz, Michael Lublow,
Adv. Energy Mater.2016.
DOI: 10.1002/aenm.201502314