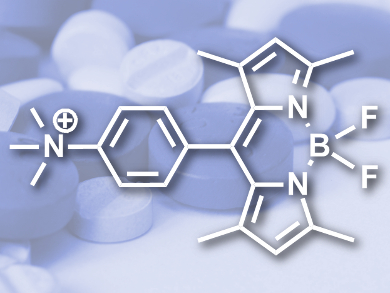
Boron-dipyrromethenes (BODIPYs) are well known for their fluorescent properties and are often utilized as part of cellular reporter probes. However, little is known about the biological activity of the BOPIDY core in its own right.
Ayşe Gürek, Fabienne Dumoulin, and colleagues, Gebze Technical University, Turkey, together with Hong Boon Lee and colleagues, University of Malaya, Kuala Lumpur, Malaysia, synthesized a simple BODIPY containing a trimethylammonium group for water solubility (pictured). Quaternary ammonium groups are often hallmarks of antimicrobial compounds.
The team tested the BODIPY for its antibacterial properties in four strains of Staphylococcus, including two methicillin-resistant S. aureus (MRSA) strains. The substance compared well against vancomycin, often the drug of last resort against MRSA, and ampicillin, a widely used antibiotic. In its more familiar role as a fluorescent dye, the BODIPY could be directly observed entering the bacteria.
Novel antibiotics are in dangerously short supply. If eventually realized as a clinical alternative to traditional antibiotics, BODIPYs would represent an unusual class of pharmacophore. Structure-activity relationship studies on BODIPY derivatives are underway and their mechanism of action has yet to be determined.
- Antimicrobial activity of a quaternized BODIPY against Staphylococcus strains,
Duygu Aydın Tekdaş, Geetha Viswanathan, sevinc zehra topal, Chung Yeng Looi, Won Fen Wong, Grace Min Yi Tan, Yunus Zorlu, Ayse Gul Gurek, Hong Boon Lee, Fabienne Dumoulin,
Org. Biomol. Chem. 2016.
DOI: 10.1039/C5OB02477C