There are many very useful transition metal-catalyzed C–H cross-coupling reactions. However, metal-free processes that generate C–C bonds remain a challenge.
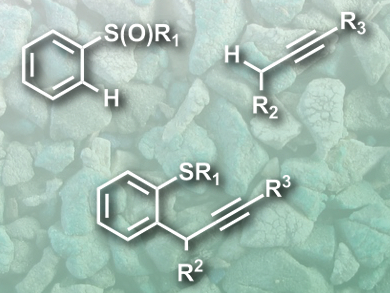
David J. Procter and colleagues, University of Manchester, UK, have developed a simple metal-free coupling reaction of arenes and alkynes, directed by a sulfoxide group at the arene. This group (pictured top left) captures and activates the alkyne (pictured top right), and controls the reactions regioselectivity.
A key step in the reaction is the formation of an alkenyl sulfonium salt, which is deprotonated and undergoes a sigmatropic rearrangement and rearomatization to give the coupling product (pictured bottom). The reaction proceeds under mild conditions, with high yields, and good functional group tolerance. The products are challenging targets for the usual metal-mediated methods and can be useful scaffolds for the synthesis of bioactive molecules.
- Metal-Free CH–CH-Type Cross-Coupling of Arenes and Alkynes Directed by a Multifunctional Sulfoxide Group,
José A. Fernández-Salas, Andrew J. Eberhart, David J. Procter,
J. Am. Chem. Soc. 2016.
DOI: 10.1021/jacs.5b12579



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)