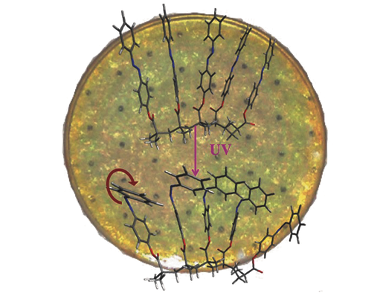
A so called solar thermal fuel (STF) absorbs sunlight and undergoes a molecular transition to a different isomer, which is higher in energy. If energy is needed, a catalyst is used to reverse the photochemical transition and heat is released. STFs are mainly available in the solution-state and suffer either from only modest energy storage capacity or from low cyclability.
Jeffrey C. Grossmann, Massachusetts Institute of Technology (MIT), Cambridge, MA, USA, and his colleagues developed a solid-state STF platform, where they use an alkyl-chain backbone with an azobenezene homopolymer as side-chain. To bring the polymer into a solid state, it is dissolved in toluene on a quartz template, which is spun to dry the solvent and to apply the polymer uniformly. In order to achieve higher heat release per quartz unit area the process is repeated several times.
By illumination with UV-light the trans-states of the azobenezene monomers are converted to the cis-state by a rotation of azobenzenes about their N=N double bond. In this state the energy is stored. Resulting STF films are able to store 30 Wh kg–1 and show a macroscopic heat release with a temperature difference of over 10 °C within several tens of seconds.
- Solid-State Solar Thermal Fuels for Heat Release Applications,
David Zhitomirsky, Eugene Cho, Jeffrey C. Grossman,
Adv. Energy Mater. 2015.
DOI: 10.1002/aenm.201502006