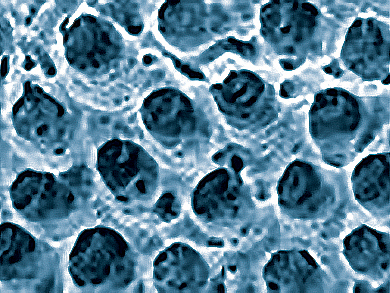
Dentin, a bone-like material that forms the crowns and roots of teeth, contains no living cells. It does not remodel or heal, yet it holds up under a lifetime of repeated impact and stress. Dentin structures contain a fibrous matrix with mineralized collagen fibers arranged in layers oriented orthogonally to tubules that run throughout the structure. Apatite mineral crystals embedded in the collagen fibers have a preferred orientation of their c-axes along the collagen fiber axis.
Paul Zaslansky, Charité−Universitätsmedizin, Berlin, Germany, and colleagues found that crystals with their c-axes aligned with the collagen fibers were more compressed along the c-axis than crystals in other orientations. The compressed apatite particles stiffen the fibers by increasing the amount of tensile stress needed to initiate and propagate cracks. The compressive stress was attributed to strong interactions between the mineral particles and collagen fibers.
The researchers suggest that the stresses are produced at an early stage of biomineral formation. They proposed a mechanism where water is expelled from the matrix, causing the collagen fibers contract, and placing stress on the tightly attached mineral particles.
- Compressive Residual Strains in Mineral Nanoparticles as a Possible Origin of Enhanced Crack Resistance in Human Tooth Dentin,
Jean-Baptiste Forien, Claudia Fleck, Peter Cloetens, Georg Duda, Peter Fratzl, Emil Zolotoyabko, Paul Zaslansky,
Nano Lett. 2015.
DOI: 10.1021/acs.nanolett.5b00143