Silicium dioxide is one of the most abundant and well-studied compounds on earth. The chemistry of SiO2 in its molecular form and other simple silicon oxides, however, is essentially unknown. This is due to their highly reactive Si=O bonds and their tendency towards oligomerization.
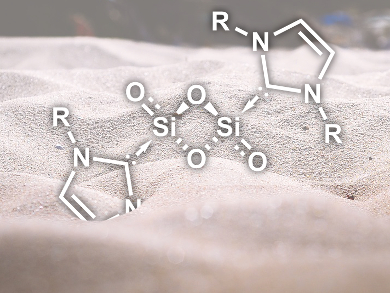
Gregory H. Robinson, University of Georgia, Athens, USA, and co-workers have succeeded in stabilizing Si2O3 and Si2O4 with bulky N-heterocyclic carbene ligands (NHCs). Starting from the stabilized disilicon coumpound NHCDipp–Si=Si–NHCDipp (Dipp = diisopropylphenyl), oxidization with N2O resulted in NHCDipp–Si2O3–NHCDipp. Reaction of the same complex with O2 gave NHCDipp–Si2O4–NHCDipp (pictured) as a product.
The researchers have characterized the compounds by 1H and 29Si NMR spectroscopy as well as X-ray crystallography. The bonding situation was investigated with quantum chemical calculations, which showed zwitterionic resonance structures as the major contributors and only a minor double bond character of the terminal Si–O bonds. Using bulky NHC ligands as steric “shields” thus allows the stabilization of elusive main-group oxides that would otherwise oligomerize.
- Stabilization of elusive silicon oxides,
Yuzhong Wang, Mingwei Chen, Yaoming Xie, Pingrong Wei, Henry F. Schaefer, Paul von R. Schleyer, Gregory H. Robinson,
Nat. Chem. 2015.
DOI: 10.1038/nchem.2234