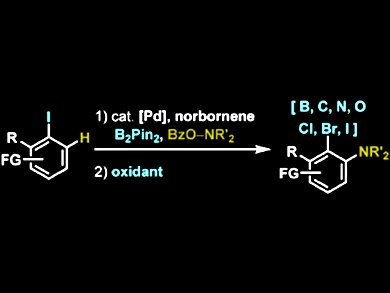
2,3-disubstituted anilines can be found in a variety of biologically active molecules including pharmaceuticals such as Viramune, Revlimid, Deactolisib. However, the current palladiumcatalyzed, norbornene-mediated 1,2-bisfunctionalization methodologies show functional group limitations at the ipso position.
Tobias Ritter and colleagues, Harvard University, Cambridge MA, USA, have succeeded in the first general ortho-amination/ipso-functionalization of aryl iodides. In this simple, two-step procedure, ipso-C−heteroatom bonds are formed in a Pd-catalyzed, norbornene-mediated ortho-C−H amination of aryl iodides. The Pd(II) intermediates are tapped with B2Pin2. The C−B bond is used as the key element to access ipso C−B, C−C, C−N, C−O, C−Cl, C−Br, and C−I bonds without the remains of a coordinating directing group.
The researchers have shown that their method provides easy access to many 2,3-disubstituted anilines and see their method as a modular strategy for the synthesis of substituted anilines.
- Modular C–H Functionalization Cascade of Aryl Iodides,
Hang Shi, David J. Babinski, Tobias Ritter,
J. Am. Chem. Soc. 2015.
DOI: 10.1021/jacs.5b01082