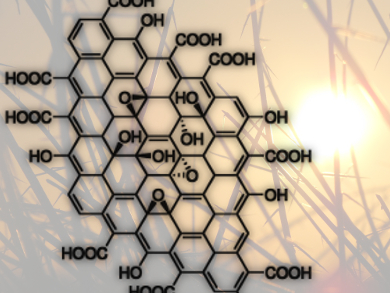
Graphene oxide (GO), a yellow solid, is an important precursor to graphene and has many promising characteristics itself. Wen-Che Hou and Richard G. Zepp, U.S. Environmental Protection Agency, Athens, GA, USA, and colleagues have investigated how GO (pictured) reacts under environmentally relevant sunlight conditions.
The researchers found that the oxide first undergoes a fast photoreaction, where GO disproportionates to fragmented photoproducts similar to reduced GO (rGO), low molecular-weight polycyclic aromatic hydrocarbon species, and CO2. The resulting rGO species are considerably less photoreactive and persist even after exposure equivalent to two months of natural sunlight.
The persistent photoproducts potentially have eco-toxicological effects distinct from those of GO. They might accumulate in the environment and likely could only be removed by environmental processes other than direct photolysis.
- Photochemical Transformation of Graphene Oxide in Sunlight,
W. Hou, I. Chowdhury, D. G. Goodwin, W. M. Henderson, D. H. Fairbrother, D. Bouchard, R. G. Zepp,
Environ. Sci. Technol. 2015.
DOI: 10.1021/es5047155




Good article.