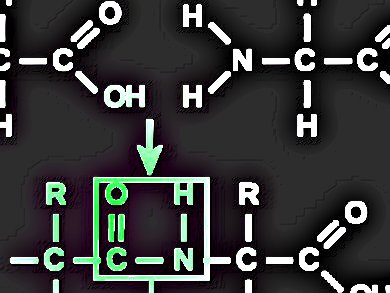
The synthesis of peptides, that is, the formation of amide bonds between amino acids and/or peptide fragments, is usually carried out in N,N-dimethylformamide (DMF). However, the use of DMF may be limited considering its overall toxicity, especially after being listed as a restricted chemical by the Commission of the European Union under its regulation on Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).
A research team led by Ayman El-Faham, Alexandria University, Alexandria, Egypt, and Fernando Albericio, Institute for Research in Biomedicine, Barcelona, Spain, has found that tetrahydrofuran (THF) and acetonitrile (ACN), two polar aprotic solvents, can replace DMF as relatively “greener” solvents. In a number of model peptide syntheses in both solution and solid phases, THF and ACN prove to be more efficient than DMF, in terms of product yields and purity. A major advantage of THF and ACN is that they can better suppress the formation of byproducts, especially through racemization.
These findings may not only improve the performance of peptide syntheses, but also lower their environmental impact.
- Peptide Synthesis Beyond DMF: THF and ACN as Excellent and Friendlier Alternatives
Fernando Albericio, Yahya Jad, Gerardo Acosta, Sherine Khattab, Beatriz G. De la Torre, Thavendran Govender, Gert Kruger, Ayman El-Faham
Org. Biomol. Chem. 2014.
DOI: 10.1039/C4OB02046D