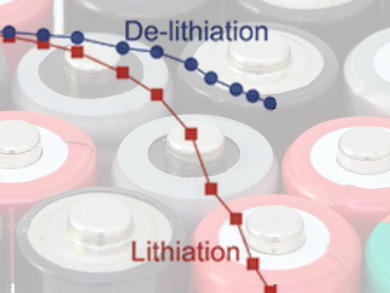
The rate capability of lithium-ion batteries is mainly determined by the architecture of the anodes and the electrochemical reaction rates of the active materials. The forward and backward reaction rate for reversible electrochemical reactions are not necessarily identical: reactions depend on concentration of reactants and products, mass transfer and charge transfer rates.
Nancy Dudney, Oak Ridge National Laboratory, USA, and colleagues show that silicon nanofilm anodes exhibit an asymmetric rate performance: 72 % of the available capacity is delivered during de-lithiation under a high current density in 22 s, whereas only 1 % of lithiation can be delivered during the same time. Using a mathematical model of single-ion diffusion, they concluded that the asymmetric behavior is caused by the potential-concentration profile as well as by the difference in chemical diffusion coefficients.
The rate performance of lithium-ion battery electrodes should be re-evaluated for charge and discharge independently. This is also necessary since in a real battery, the charge and discharge protocols are not always the same: charging protocols are controlled by manufactures, but the discharging process depends on the usage by the costumer.
- Asymmetric Rate Behavior of Si Anodes for Lithium-Ion Batteries: Ultrafast De-Lithiation versus Sluggish Lithiation at High Current Densities,
Juchuan Li, Nancy J. Dudney, Xingcheng Xiao, Yang-Tse Cheng, Chengdu Liang, Mark W. Verbrugge,
Adv. Energy Mater. 2014.
DOI: 10.1002/aenm.201401627