A promising alternative to the classic graphite anode material in Lithium-ion (Li-ion) batteries is tin oxide, due to its high theoretical capacity. In the real world, the application is hindered by poor cycling stability and rate performance. The reasons are large volume changes (up to 300 %) during lithiation and delithiation, resulting in the detachment of SnO2 particles and their agglomeration in large inactive clusters.
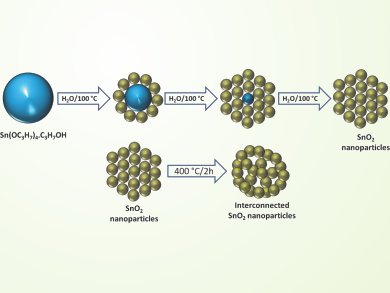
Vilas Pol, Purdue University, West Lafayette, USA, and colleagues established a synthesis of ordered interconnected SnO2 nanoparticles. Addition of the solid alkoxide precursor tin tetraisopropoxide into boiling water results in the diffusion-controlled hydrolysis to produce uniform SnO2 nanoparticles. Further heat-treatment (400 °C for 2 h) results in the self-assembly into an ordered and interconnected network of SnO2 particles.
This network demonstrates excellent Li-ion specific capacities, rate performance and long-term cycling stability, even after 100 cycles.
The unique structure of this anode with its mesoporisity and interconnection of SnO2 nanoparticles allows the accommodation of volume changes during Li-ion storage and thereby prevents anode degeneration.
- Ordered Network of Interconnected SnO2Nanoparticles for Excellent Lithium-Ion Storage
Vinodkumar Etacheri, Gulaim A. Seisenbaeva, James Caruthers, Geoffrey Daniel, Jean-Marie Nedelec, Vadim G. Kessler, Vilas G. Pol.
Adv. Energy Mat. 2014.
DOI: 10.1002/aenm.201401289



What is the easiest way laboratory For the production of carbon dioxide