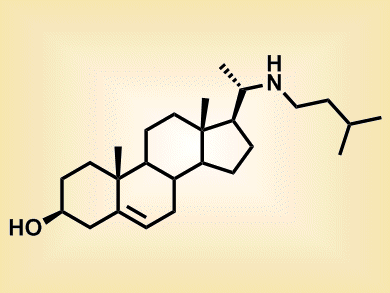
The development of numerous tumors is driven by the aberrant activation of a trans-membrane protein called smoothened (Smo). The extracellular portion of this molecule contains two important domains. The first one, site A, is targeted by small inhibitors such as the alkaloid cyclopamine. The second one, site B, is the binding site for oxysterols, a family of oxygenated cholesterol derivatives that activate Smo allosterically.
According to Daniel Nedelcu and colleagues, Harvard Medical School, Boston, USA, smoothened inhibition can be achieved not only by targeting site A but also by antagonizing oxysterol’s effects. The researchers synthesized 22-azacholesterol (pictured) and demonstrated that this compound competes with oxysterols for binding to Smo at site B. By doing so, 22-azacholesterol blocked Smo activation through an allosteric mechanism.
The use of 22-azacholesterol might, thus, be a novel and effective strategy to target Smo and prevent its aberrant activation during the development of cancer.
- Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling,
Daniel Nedelcu, Jing Liu, Yangqing Xu, Cindy Jao, Adrian Salic,
Nat. Chem. Biol. 2013, 9, 557–564.
DOI: 10.1038/nchembio.1290