Alkaloids are often potentially useful drug leads with complex physiological activity. Unfortunately, they are also often complex in structure, with many different chemical groups all putatively containing multiple stereo centers. Rare is the synthesis that a medicinal chemist can describe as concise in this realm.
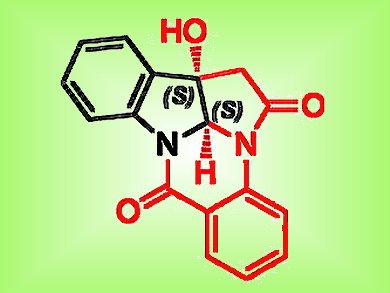
Vipin Nair and colleagues, National Institute of Pharmaceutical Education and Research, Mohali, Punjab, India, have devised just such a concise total synthesis for a newly discovered alkaloid from the roots of the plant Isatis indigotica. This species has been used in traditional medicine and recent studies revealed that the alkaloid with the pyrrolo[2,3-b]indolo[5,5a,6-b,a]quinazoline skeleton displays several different types of biological activity, including antiviral activity against influenza.
The team exploited an asymmetric acetate aldol reaction on tryptanthrin, which was mediated by a chiral auxiliary. The resulting adduct formed by this reaction could then be converted into the final product using a one-pot reductive cyclization/transamidation reaction with nickel chloride and sodium borohydride in methanol.
- Total Synthesis of a Pyrroloindoloquinazoline Alkaloid,
Digvijay Gahtory, Mangilal Chouhan, Ratnesh Sharma, Vipin A. Nair,
Org. Lett. 2013.
DOI: 10.1021/ol401709t