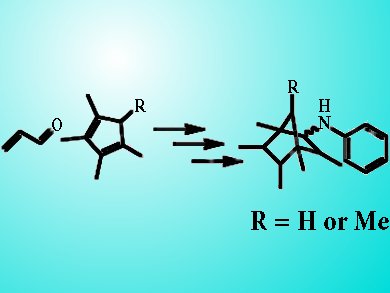
Leroy Cronin and colleagues, University of Glasgow, UK, used a 3D printer to create a reactor for a three-step organic synthesis. The sealed L-shaped reactor contained the reagents and the catalyst. The reaction steps were performed by simply rotating the device so that the reaction mixture flowed through successive environments under gravity, without the need for any pumps or liquid-handling prior to product retrieval from the reaction ware in a pure form. The sequence began with a Diels–Alder cyclisation followed by formation of an imine and then hydrogenation of the imine to the corresponding secondary amine. A purification step for the synthesis ia also integrated into the chambers of the sealed reactor.
The data for the reactor are open source, allowing for the reproduction of the reactive system in any laboratory that has a 3D printer.
- Combining 3D printing and liquid handling to produce user-friendly reactionware for chemical synthesis and purification,
Philip J. Kitson, Mark D. Symes, Vincenza Dragone, Leroy Cronin,
Chem. Sci. 2013.
DOI: 10.1039/C3SC51253C