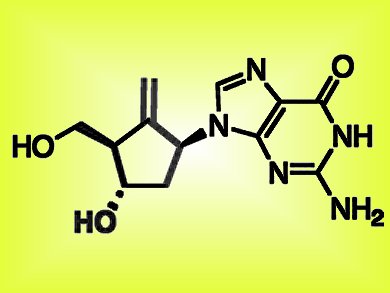
Hepatitis B is one of the most common infections worldwide, affecting around two billion people. A number of drugs exist to treat this infection, which untreated can result in cirrhosis of the liver or liver cancer. The patent of one such drug, entecavir (pictured), which shows no significant adverse effects and has a low risk of inducing long-term resistance, expires in 2015 in the US making this drug attractive as a generic target.
A new route to entecavir, developed by Xavier Ariza, Jaume Farras̀, and colleagues, Universitat de Barcelona, and colleagues from Esteve Química, both Spain, starts from commercially available acyclic precursors. The key points of this 11-step synthesis are a stereoselective boron−aldol reaction, a Cp2TiCl-catalyzed intramolecular radical addition of an epoxide to an alkyne, and coupling with a purine derivative by a Mitsunobu reaction. The products in the final steps are purified by crystallization making this attractive sequence amenable to scale-up.
- Total Synthesis of Entecavir,
Javier Velasco, Xavier Ariza, Laura Badía, Martí Bartra, Ramon Berenguer, Jaume Farràs, Joan Gallardo, Jordi Garcia, Yolanda Gasanz
J. Org. Chem. 2013.
DOI: 10.1021/jo400607v