Xavier Creary, University of Notre Dame, Indiana, USA, has made a square-shaped carbene, 3-trimethylsilylcyclobutylidene by pyrolysis of a sodium salt derivative of 3-trimethylsilylcyclobutanone. Labeling studies reveal how this unstable species undergoes a rearrangement to form what looks like a folded square with a bridge. This occurs through a 1,3-hydrogen migration to the carbenic center rather than the suspected 1,3-silyl migration.
Silyl groups have been well studied as tools for controlling reactions and rearrangements, but on this occasion they seem not to be the group orchestrating the movement.
- 3-Trimethylsilylcyclobutylidene. The gamma-Effect of Silicon on Carbenes,
Xavier Creary,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja400747u
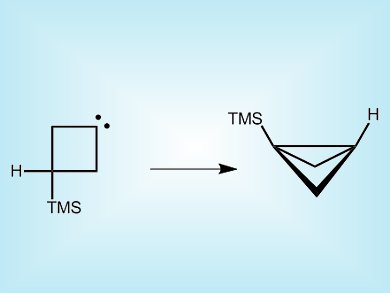




The figure of the product drawn here has one carbon too many (Butane can not go to pentane.). The top bridge should not be bent, but straight.
You are quite right, the original picture for this article did indeed contain an extra carbon. We have corrected the structure, thank you for pointing out our mistake. The ChemViews team.