Sirtuins are enzymes which regulate numerous biological processes, including metabolism and life-span. Most of these molecules are considered nicotinamide adenine dinucleotide (NAD)-dependent deacetylases as they remove acetyl groups from lysine residues present on target proteins. Nevertheless, some sirtuins, such as SIRT6, show a very weak deacetylase activity.
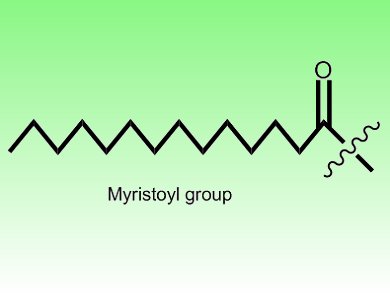
Hong Jiang, Cornell University, New York, USA, and colleagues revealed the reason for this incongruity. The scientists demonstrated that SIRT6’s preferred substrates are not acetyls, but are instead long-chain fatty-acyls such as myristoyl or palmitoyl groups. As a consequence, while SIRT6 only weakly deacetylates lysine residues, it efficiently removes their myristoyl groups. In doing so, this enzyme controls the secretion of tumor necrosis factor α, an important inflammatory molecule. SIRT6, therefore, has a previous unrecognized enzymatic activity which plays important biological roles.
- SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine,
H. Jiang, S. Khan, Y. Wang, G. Charron, B. He, C. Sebastian, J. Du, R. Kim, E. Ge, R. Mostoslavsky, H. C. Hang, Q. Hao, H. Lin,
Nature 2013, 496 (7443), 110–113.
DOI: 10.1038/nature12038