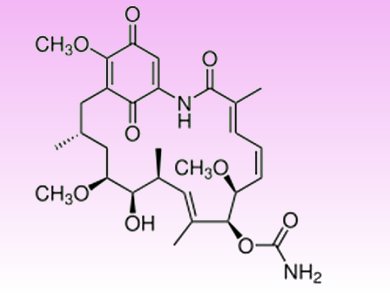
Heat shock protein 90 (HSP90) is an important anti-cancer target as it stabilizes proteins involved in tumor growth. While geldanamycin, a benzoquinone ansamycin antibiotic (pictured), and its synthetic analogs potently inhibit HSP90, they induce hepatotoxicity and, thus, have a limited clinical use.
This issue promoted Russell Kitson, University of Nottingham, UK, and colleagues to develop strategies to decrease geldanamycin-induced liver damage. Since this side effect has been attributed to the reaction of biological nucleophiles at the 19-position of the quinone ring, the researchers synthesized novel 19-substituted benzoquinone ansamycins. These compounds retained the potency of their parent quinones but showed less toxicity when tested in vitro as the C-19 substitutions prevented the reaction with thiol nucleophiles. The new geldanamycin derivatives are, therefore, promising anti-cancer drugs.
- Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90,
R. R. Kitson, C. H. Chang, R. Xiong, H. E. Williams, A. L. Davis, W. Lewis, D. L. Dehn, D. Siegel, S. M. Roe, C. Prodromou, D. Ross , C. J. Moody,
Nat. Chem. 2013, 5 (4), 307–314.
DOI: 10.1038/nchem.1596