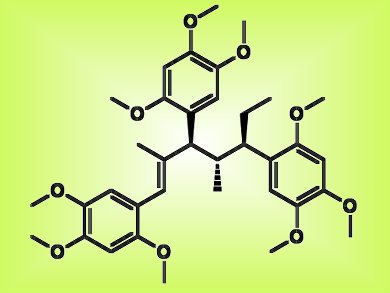
A class of complex polyphenolic natural products was previously reported to be potent antidiabetic agents. A synthetic route to these compounds, tatanans A, B, and C, could prompt effective new treatments for diabetes if proven.
The total synthesis of tatanan A (pictured), which has three consecutive tertiary stereocenters, was achieved in 13 steps with a series of sequential [3,3]-sigmatropic rearrangements in 13 % yield by Brian Miller, Armen Zakarian, and colleagues from the University of California, Santa Barbara, and Florida State University, both USA. Tatanans B and C were obtained in 12 steps, including an aromatic group differentiation step, in 4 and 8 % yields, respectively.
Disappointingly, this study showed tatanans A–C had no antidiabetic activity. The origin of the discrepancy is unclear, but perhaps another natural product responsible for the biological effects was co-isolated during the original study.
- Enantioselective synthesis of tatanans A–C and reinvestigation of their glucokinase-activating properties,
Qing Xiao, Jeffrey J. Jackson, Ashok Basak, Joseph M. Bowler, Brian G. Miller, Armen Zakarian,
Nat. Chem. 2013.
DOI: 10.1038/nchem.1597