Archaebacteria in the intestines of cows and in oxygen-depleted water bodies and soil produce the greenhouse gas methane from hydrogen and carbon dioxide as well as from other carbon compounds. Hydrogenases, which are found in the cell of archaebacteria, split hydrogen into protons and electrons which are then transferred to carbon dioxide in a process involving several steps.
Janet Vonck and colleagues, Max Planck Institute of Biophysics, Frankfurt, and Seigo Shima and collegaues, Max Planck Institute for Terrestrial Microbiology, Marburg, both Germany, have discovered the structure of the F420-reducing [NiFe]-hydrogenase, referred to as Frh, and the location of its docking site for the coenzyme F420. So far, the structure of the enzymes from only one of five groups into which the nickel-iron hydrogenases are divided, was known.
It was already known that Frh binds the coenzyme F420 in a special additional subunit. The scientists have decoded key aspects of the enzyme structure with the help of cryo-electron microscopy. They foze the proteins in a thin layer of ice so that the biopolymers are immobilised. Using an electron microscope, the researchers then recorded numerous images of the proteins in different orientations. Then the images were combined computionally to calculate the three-dimensional structure of the complex.
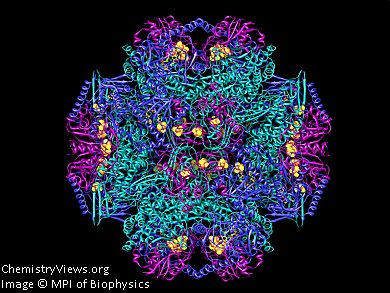
Frh forms large tetrahedral complexes. Three different subunits form a functional unit (pictured). A total of twelve of these trimers combine to form a macromolecule. Why twelve trimers join forces is not yet understood.
By comparing the structure of the enzyme without F420 with the structure to which the coenzyme was bound, the pocket in which the coenzyme binds was identified.
The findings could help to better understand the influence of methane-producing archaebacteria on the climate and in the development of synthetic catalysts for hydrogen production. The reactions, in which hydrogenases split hydrogen into proteins and electrons, can be reversed so that the enzymes produce hydrogen from protons and electrons.
Image: Molecular structure of Frh proteins; one blue, one green and one violet subunit together form a trimer
© MPI of Biophysics
- Max Planck Gesellschaft, Munich, Germany