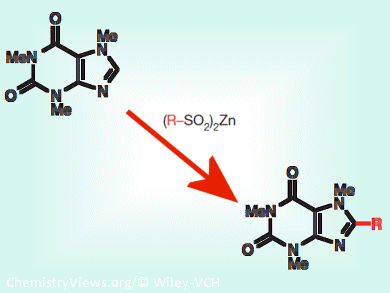
Heterocycles, molecules containing a ring where at least one atom is not a carbon, play important roles in medicinal chemistry as they constitute a wide range of drugs. Yuta Fujiwara, Scripps Research Institute, USA, and colleagues demonstrated that ten different zinc bis(alkanesulphinate) salts can be used as a chemical toolkit to incorporate diverse chemical groups into heterocycles and, thus, generate novel chemical compounds for drug discovery.
Zinc bis(alkanesulphinate) salts reacted at heterocycles’ carbon-hydrogen bonds, transforming them into carbon-carbon bonds by attaching the alkyl group of the salts. This conversion produced novel heterocycles bearing pharmaceutically important groups such as the fluoroalkyl group, required to improve the half-life of drugs. The reaction, moreover, could proceed in different solutes (organic solvents, water, cellular fluids), it did not induce substrate decomposition, and it could be sequentially performed.
- Practical and innate carbon–hydrogen functionalization of heterocycles,
Y. Fujiwara, J. A. Dixon, F. O’Hara, E. D. Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herlé, N. Sach, M. R. Collins, Y. Ishihara, P. S. Baran,
Nature 2012, 98(492), 95–100.
DOI: 10.1038/nature11680