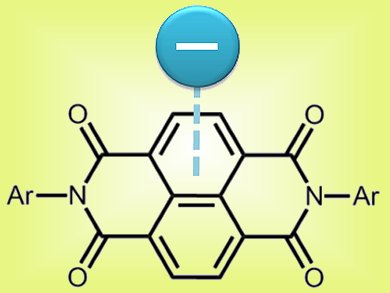
Anion-π interactions have been discovered only recently. They complement anionic non-covalent interaction chemistry, which was for a long time considered to be restricted to Lewis acid coordination and hydrogen bonding. However, unlike Lewis acid coordination and hydrogen bonding, the mechanism of these anion-π interactions is not fully understood.
Sourav Saha and colleagues, Florida State University, Tallahassee, USA, analyzed the interactions of many anions of different types with electron-deficient π-systems. They concluded that, given a specific electron-deficient system, the strength of interaction depends only on the Lewis basicity of the anion in an aprotic solvent. Using various spectroscopic and electrochemical techniques, they could show that anion-π interactions cover a whole range of mechanisms, from weak non-chromogenic interactions to anion-induced chromogenic charge-transfer and photoinduced and thermal electron transfer.
This discovery might possess multiple applications, for example, in analytical chemistry, where it could be used to detect toxic fluoride by its chromogenic reaction with π-deficient systems.
- Boundaries of Anion/Naphthalenediimide Interactions: From Anion−π Interactions to Anion-Induced Charge-Transfer and Electron-Transfer Phenomena,
S. Guha, F. S. Goodson, L. J. Corson, S. Saha,
J. Am. Chem. Soc. 2012, 134 (33), 13679–13691.
DOI: 10.1021/ja303173n