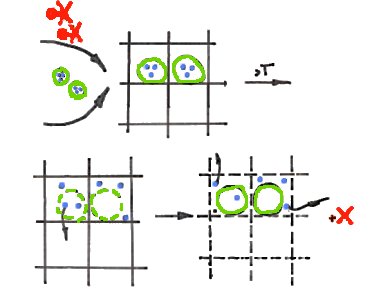
To develop a model delivery platform with a tunable release rate, Jan H. van Esch and colleagues, Delft University of Technology, The Netherlands, designed a system comprised of three major components: a hydrogelator, a proteolytic enzyme, and a phospholipid. The low-molecular-weight hydrogelator, based on a cyclohexane-tris-amide core, is covalently connected to the fluorogenic molecule 6-aminoquinoline (6-AQ) via an enzymatically hydrolyzable amide linker.
Adding to a solution of the hydrogelator (red) in DMSO an aqueous solution of the enzyme α-chy (blue) loaded in liposomes (green), results in the formation of a gel network with embedded liposomes.
Briefly heating the gel at the Tm of the liposomes results in partial liberation of the enzyme into the gel matrix. Through enzymatic hydrolysis of the amide bond the fluorescent 6-AQ is released.
Crucially, the amount of liberated enzyme, and thus the fluorophore release rate, can be tuned by the heating time of the platform. In future work, other enzymes, catalysts, and associated addressable chemical bonds might be applied to enhance biocompatibility and selectivity. In combination with drug-carrying gelators, such a concept could find use in future applications in personalized medicine or medical implants, where the drug release rate can be tuned before intake of the medicine or by locally induced hyperthermia.
- A Self-Assembled Delivery Platform with Post-production Tunable Release Rate,
Job Boekhoven, Mathijs Koot, Tim A. Wezendonk, Rienk Eelkema, Jan H. van Esch,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja3051876