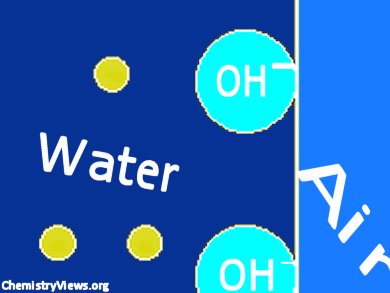
Rayleigh and subsequently Bohr developed the oscillating jet method for measuring surface tension. Schmidt et al. used this method and found in 1926 that the surface tension of a freshly formed, pristine water/air interface is 80−100 mN/m. This is temperature dependent. The surface tension relaxes to its equilibrium value in about 1 ms.
Angus Gray-Weale, University of Melbourne, Victoria, Australia, and colleagues examined three possible explanations for this millisecond relaxation time:
- the diffusion of surfactant contaminants from the aqueous phase to the surface,
- the reorientation of surface water molecules’ dipole moments, and
- the buildup of a charged surface layer of hydroxide ions.
They found that the surface tension of water is the tension of the surface spontaneously charged by a flow of hydroxide to the surface. Through a combination of autolysis of water and diffusion of hydroxide ions to the interface, the surface tension relaxes. The formation of the charged double layer lowers the surface tension, just like the adsorption of typical soap-like amphiphilic surfactants. The relaxation time was found to be salt and pH dependent.
The surface tension of water is used as a standard experimental value and was thought to be the tension of the pristine air/water interface.
- The Surface Relaxation of Water,
Maoyuan Liu, James K Beattie, Angus Anthony Gray-Weale,
J. Phys. Chem. B 2012.
DOI: 10.1021/jp211810v