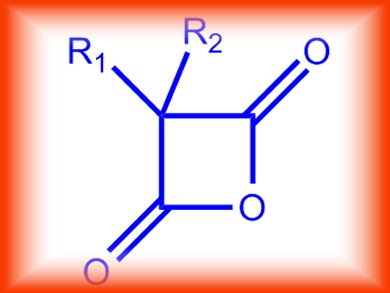
Malonic anhydrides (pictured) are used in organic synthesis because of the ease with which they can be derivatized to various monoesters and monoamides for further conversion into pharmaceuticals, natural products, and other biologically active compounds. Conversely, their rapid room temperature decomposition to a ketene and carbon dioxide is also of interest from the perspective of their mechanistics.
Researchers at the University of California—San Diego, La Jolla, USA, have now demonstrated that the 2 + 2 cycloadditions and cycloreversions of these compounds take place via a concerted but asynchronous mechanism rather than a controversial stepwise process. The results provide definitive answers to the long-standing questions regarding the rapidity of decomposition and the substituent effects on reactivities.
- Decomposition of Malonic Anhydrides,
Charles L. Perrin, Agnes Flach, Marlon N. Manalo,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja301867s