Contrary to the rule that heritable information flows from DNA and RNA to protein, but never from protein to any other molecule, prions are able to transmit information from one molecule to another through the transmission of their misfolded shape, with devastating consequences in diseases such as mad cow disease and Creutzfeldt-Jakob disease. The broader implications of this mechanism are not well understood.
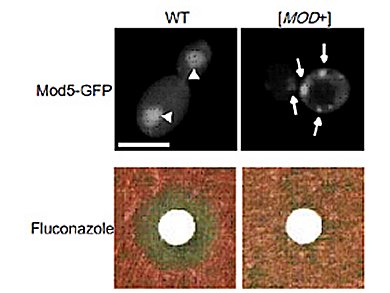
Researchers at the RIKEN Brain Science Institute (BSI), Japan, screened a wide range of different genes in budding yeast for previously-undiscovered prions. Out of 6000 genes screened, they found a new yeast prion protein “Mod5” with the unusual property that it lacks the glutamine and asparagine-rich amino acid sequences characteristic of other yeast prions. Sequences like these are thought to contribute to forming amyloid aggregates, the mechanism by which prions propagate.
Despite lacking these sequences, Mod5 forms amyloid aggregates just like other yeast prions. The researchers showed that Mod5 aggregates actually help the yeast, by granting it cellular resistance to antifungal agents. This advantage is so important that the yeast actually increases prion conversion when the pressure is on, as the researchers found when they applied antifungal drugs to the yeast.
Image: ©: RIKEN Brain Science Institute
- A Yeast Prion, Mod5, Promotes Acquired Drug Resistance and Cell Survival Under Environmental Stress,
Genjiro Suzuki, Naoyuki Shimazu and Motomasa Tanaka,
Science 2012.
DOI: 10.1126/science.1219491