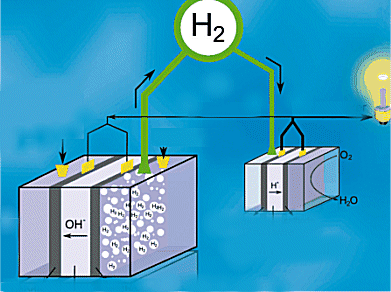
Hydrogen is not an abundant gas on Earth. For it to become the fuel of a future green economy we will need to make it cheaply and easily. Fuel cells, however, need highly pure hydrogen gas something that is neither cheap nor easy to produce.
Now, a catalyst that uses just trace amounts of platinum can generate very pure hydrogen gas in the water-gas-shift reaction according to US research. The catalyst is robust and effective even at low temperature and the team is now using experiment and first-principles theory to identify its mode of action. That work could allow them to swap the platinum for something cheaper still.
- Alkali-Stabilized Pt-OHx Species Catalyze Low-Temperature Water-Gas Shift Reactions
Y. Zhai, D. Pierre, R. Si, W. Deng, P. Ferrin et al.,
Science 2010, 329, 1633-1636.
DOI: 10.1126/science.1192449