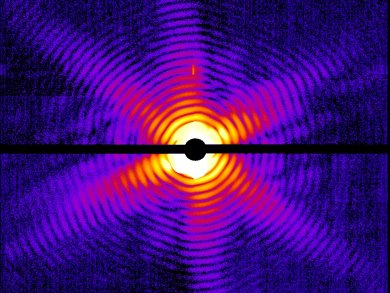
X-ray crystallography relies on the ability to grow a crystal of sufficient size. If a crystal is too small, the X-ray dose required to obtain data damages the structure. This can be a problem with some macromolecules and biological structures which do not crystallize easily.
Henry Chapman, University of Hamburg, Germany, and colleagues have used ultra-short pulses from a hard-X-ray free-electron laser, Linac Coherent Light Source, Stanford Linear Accelerator Center, USA, to collect diffraction data from the Mimivirus – the world’s largest known virus – and photosystem I – one of the largest membrane protein complexes. The short pulse time (50—70 femtoseconds) ensures the nanocrystals are not damaged before the diffraction signal can be recorded as the pulses are shorter than the time scales of most damage processes.
The authors hope this new method will allow the study of biological structures at the molecular level, including viruses, individual cells, cell organelles, and living bacteria, which are incredibly hard to study using conventional methods.
Image: © Janos Hajdu/Uppsala University
- Femtosecond X-ray protein nanocrystallography
H. N. Chapman, P. Fromme, A. Barty, T. A. White, R. A. Kirian et al.,
Nature 2011, 470, 73–77.
DOI: 10.1038/nature09750