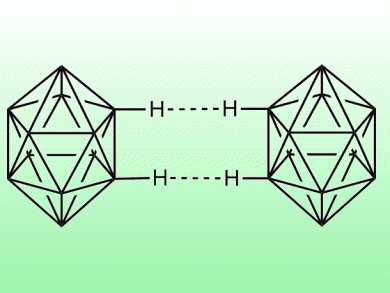
To explain high melting points and vaporization enthalpies of polyhedrane hydrocarbons would require that bonds between hydrogen atoms on neighboring molecules be much stronger than experience would suggest.
Systematic calculations now carried out by a Spanish-Israeli team on dimers of alkanes and polyhedranes show that C–H···H–C dihydrogen bonds could account for the physical observations. Such fundamental insights into seemingly uncomplicated substances continue to throw our chemical understanding into relief and ultimately will allow novel materials to emerge from research.
- Dihydrogen contacts in alkanes are subtle but not faint
J. Echeverría, G. Aullón, D. Danovich, S. Shaik, S. Alvarez,
Nature Chem. 2011.
DOI: 10.1038/nchem.1004