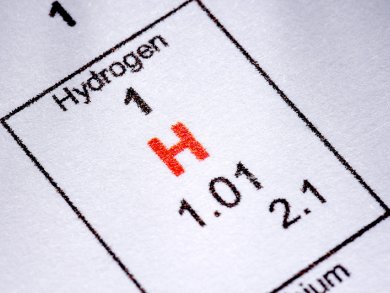
Finding safe and energy-dense hydrogen storage materials to carry the chemical energy for fuel cells is high on the green chemistry agenda. Ammonia borane has been discussed as one such material, but it releases its hydrogen at a temperature above that of boiling water, 110 °C. Now, US researchers have unlocked the crystal structure of an alternate material that gives up its H2 at a cooler 85 °C, but is nevertheless stable at room temperature.
The next step will be to test reversibility of the reaction to reveal whether diammoniate of diborane could be “refueled” and so be viable for fuel cells in vehicles.
Image: (c) Wiley-VCH
- The diammoniate of diborane: crystal structure and hydrogen release
M. Bowden, D. J. Heldebrant, A. Karkamkar, T. Proffen, G. K. Schenter, T. Autrey,
Chem. Commun. 2010, 46, 8564.
DOI: 10.1039/c0cc03249b