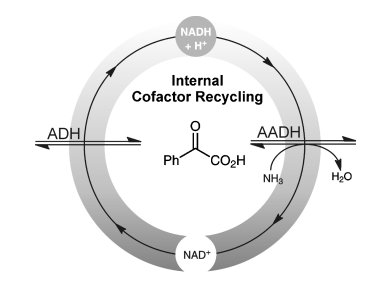
Wolfgang Kroutil and co-workers from the University of Graz, Austria, highlight the deracemization of mandelic acid to optically pure L-phenylglycine via a redox-neutral biocatalytic cascade, in their recent communication.
The procedure consisted of three steps:
- a racemization,
- an enantioselective oxidation, and
- a stereoselective reductive amination.
Racemic mandelic acid was transformed to optically pure L-phenylglycine (ee >97 %) at 94 % conversion without the requirement of any additional redox reagents in stoichiometric amounts.

(C) Wiley-VCH
- Deracemisation of Mandelic Acid to Optically Pure Non-Natural L-Phenylglycine via a Redox-Neutral Biocatalytic Cascade
V. Resch, W. M. F. Fabian, W. Kroutil
Adv. Synth. Catal. 2010, 352, 993–997.