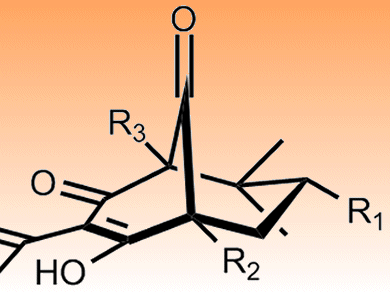
The natural products polyprenylated polycyclic acylphloroglucines (PPAPs) have a common bicyclo[3.3.1]nonane-2,4,9-trione core (pictured). This provides a rigid bicyclic framework on to which various sidechains might be added to generate new leads given the range of biological activity seen in the parent compounds. These include antimicrobial, antioxidant, anticancer and antidepressant properties.
Bernd Plietker and colleagues, University of Stuttgart, Germany, have devised a seven-step synthesis to PPAPs, hyperpapuanone, hyperibone L, epi-clusianone and oblongifolin A, originally from Clusiaceae (Guttiferae) plants. This new route allows them to add sidechains sequentially to produce trans-type derivatives.
- The total synthesis of hyperpapuanone, hyperibone L, epi-clusianone and oblongifolin A
N. Biber, K. Möws, B. Plietker,
Nature Chem. 2011.
DOI: 10.1038/nchem.1170