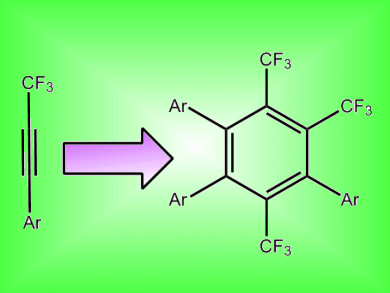
Benzene derivatives with trifluoromethyl groups have shown interesting biological activity, in part due to the presence of fluorine, a common motif in pharmaceuticals. Synthesis of substituted benzenes can be efficiently performed through [2 + 2+ 2] cycloadditions of alkynes, although examples with fluorine-containing alkynes are rare.
A ruthenium catalyst that catalyzes the [2 + 2 + 2] cycloaddition of fluorine-containing alkynes has now been reported by Motoi Kawatsura and colleagues, Tottori University, Japan. The catalyst consisted of Ru3(CO)12 coordinated with 2-(diphenylphosphino)benzonitrile. With this catalyst, CF3-substituted benzene derivatives could be made in up to 92 % yield with >98 % regioselectivity.
- Ruthenium-Catalyzed Regioselective [2 + 2 + 2] Cyclotrimerization of Trifluoromethyl Group Substituted Internal Alkynes
M. Kawatsura, M. Yamamoto, J. Namioka, K. Kajita, T. Hirakawa, T. Itoh,
Org. Lett. 2011.
DOI: 10.1021/ol1030734