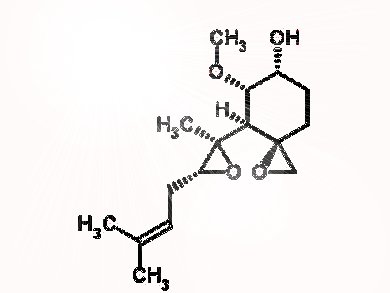
A diverse library of compounds for pharmaceutical screening can be created starting with a single reactive natural product, according to US chemists.
Researchers at Boston University started with the highly oxygenated natural product fumagillol to create a diverse compound library for physiological screening. The team used a reaction-based approach to remodel the original compound in several different ways. For instance, reactions with amines occur with excellent regiocontrol to form epoxide opening/cyclization sequences to give perhydroisoindole or perhydroisoquinoline products. These intermediates can themselves be remodelled using a cascade of reactions to generate morpholinone or bridged 4,1-benzoxazepine derivatives.
- Remodelling of the natural product fumagillol employing a reaction discovery approach,
Bradley R. Balthaser, Meghan C. Maloney, Aaron B. Beeler, John A. Porco Jr, John K. Snyder,
Nature Chem. 2011.
DOI: 10.1038/nchem.1178