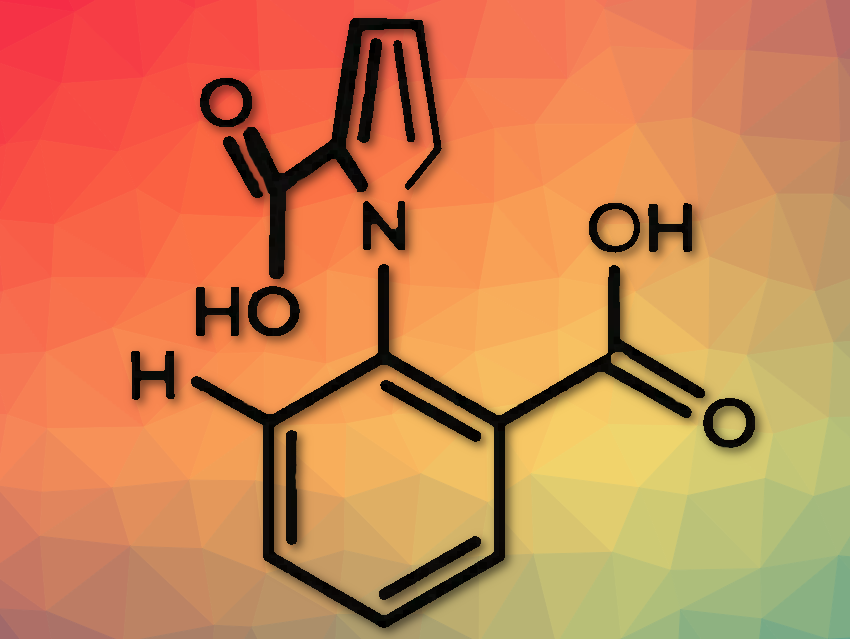
David A. Leight and colleagues, University of Manchester, Manchester, UK, and East China Normal University, Shanghai, have developed a catalysis-driven motor with the first autonomous 360° rotation in one direction around a single bond. They found that 1-phenylpyrrole-2,2′-dicarboxylic acid (pictured above) can continuously convert energy from a chemical fuel to cause repeated 360° rotation of the two aromatic rings around the covalent N–C bond that connects them. Reacting it with a carbodiimide as fuel (pictured below), constant intramolecular anhydride formation between the rings and hydrolysis of the anhydride occur.
.png)
Both reactions are kinetically controlled. This results in directional control, such that the catalysis of the carbodiimide hydration by the motor molecule continuously results in a net directional rotation about the N–C bond.
The direction of rotation is determined by the handedness of both an additive that accelerates the anhydride hydrolysis and the fuel. It can be easily reversed. The researchers found that the motor rotates at a rate of about one rotation every three hours. It makes a ‘mistake’ in direction every three to four turns. They hope that the structural simplicity of the device will enable future designs with faster rotation and fewer errors.
- Autonomous fuelled directional rotation about a covalent single bond,
Stefan Borsley, Elisabeth Kreidt, David A. Leigh, Benjamin M. W. Roberts,
Nature 2022, 604, 80–85.
https://doi.org/10.1038/s41586-022-04450-5
- Animation of the design and autonomous chemically fuelled rotation of motor molecule
Credit: Stuart Jantzen, Biocinematics