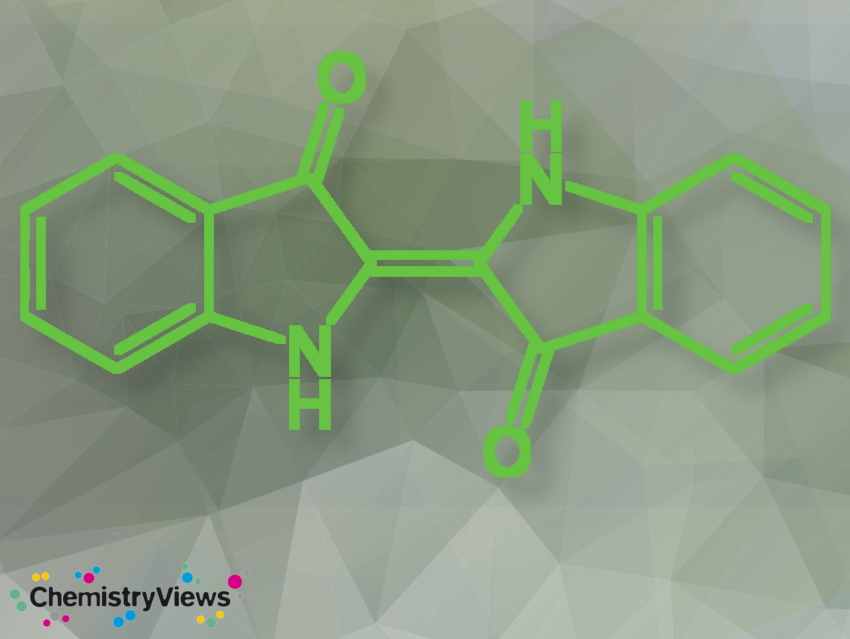
Indigo, 2-(3-hydroxy-1H-indol-2-yl)indol-3-one
Who?
Johann Friedrich Wilhelm Adolf von Baeyer (1835–1917)
When?
1878
How?
Adolf von Baeyer, a Ph.D. student of August Kekulé (1812–1896) who succeeded Justus von Liebig as Chemistry Professor at the University of Munich, Germany, identified isatin as a decomposition product of indigo.
For his first synthesis, he produced isatin from phenylacetic acid via oxindole [1,1a,1b]. Then, he chlorinated the isatin with phosphorus pentachloride (PCl5) to form isatin chloride, which he reduced with zinc in acetic acid to form indigo [2–4] (see Scheme 1). Baeyer developed this second step in collaboration with Adolph Emmerling (1842–1906) and later optimized it [5].
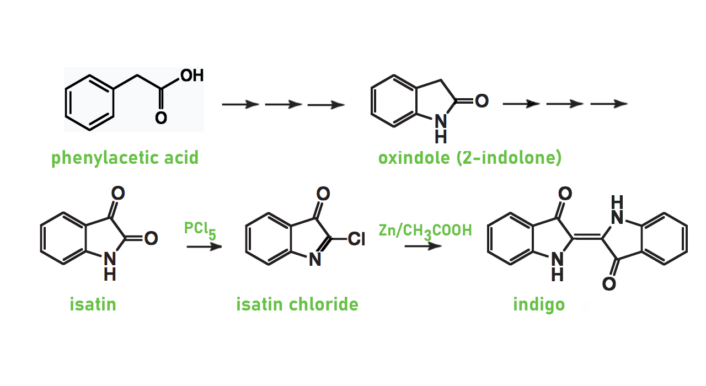
Scheme 1. First synthesis of indigo.
Between 1880 and 1882, Adolf von Baeyer developed syntheses starting from cinnamic acid [6] or o-nitrobenzaldehyde [7] (see Schemes 2 and 3).
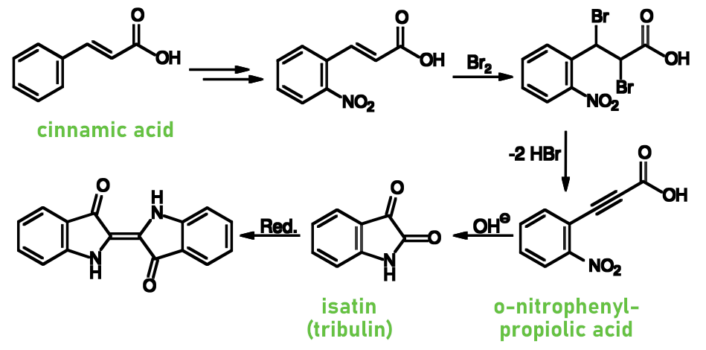
Scheme 2. Indigo synthesis [6].
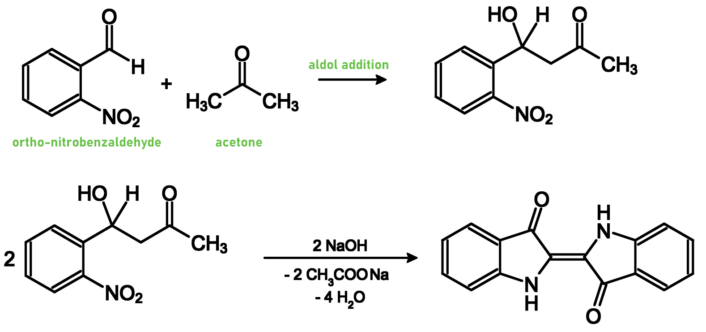
Scheme 3. Synthesis of indigo by Beayer and Viggo Beutner Drewsen (1858–1930), a Ph.D. student of Beayer [7].
While the first two syntheses could not be converted into industrial processes, BASF succeeded with the third synthesis in producing the so-called “Kleines Indigo” (small indigo). Heinrich Caro, head of research and a close friend of Adolf von Baeyer, discovered that o-nitrophenylpropiolic acid could be converted directly into indigo on fibres under mild alkaline conditions using sodium xanthogenate. Later, Hoechst also joined in, however, it was not a commercial success and three years later, production was discontinued.
Karl Heumann—First Commercial Synthesis
Karl Heumann (1850–1894), Eidgenössisches Polytechnikum in Zurich (ETH), Switzerland, developed two key synthesis routes for indigo. The first (1890) used N-phenylglycine but was abandoned due to low yields. The second (1897) converted anthranilic acid into indigo via phenylglycine-o-carboxylic acid and indoxyl and achieved much higher efficiency, enabling industrial production by BASF starting in 1897 with yields of 70–90% (see Scheme 4).
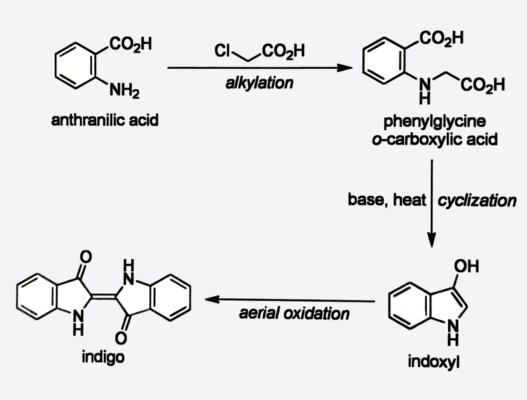
Scheme 4. Heumann Synthesis [8].
BASF produced anthranilic acid on an industrial scale. Naphthalene, a byproduct of the coal tar dye industry, was oxidized to phthalic acid using mercury sulfate as a catalyst. Reacting the phthalic acid with ammonia converted it into phthalamide, which was then transformed into anthranilic acid through the Hofmann rearrangement.
Subsequent innovations included the Heumann-Pfleger synthesis (1901). In this process, phenylglycine, produced from aniline, formaldehyde, and hydrogen cyanide, is fused with sodium amide under anhydrous, alkaline conditions (see Scheme 5 [9]). A BASF variant in 1905 replaced sodium amide with cheaper calcium oxide. By 1924, BASF transitioned to synthesizing indigo from phenylglycine nitrile derived from aniline.
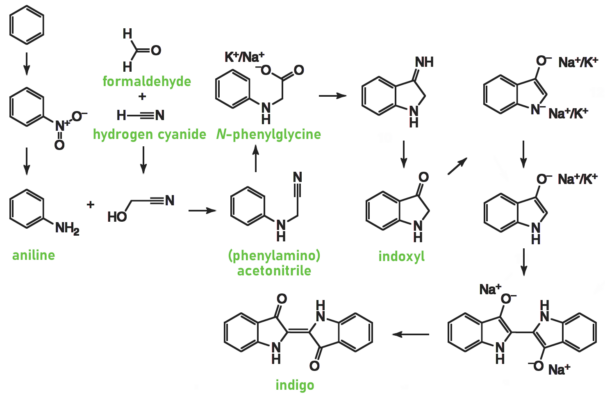
Scheme 5. Heumann-Pfleger synthesis [9].
A Very Short History of Indigo
Some of the oldest written records of dyes come from China and India. According to K. C. Nicolaou, a 5,000-year-old text from China describes recipes for dyeing silk red, black, and yellow [1].
India gave its name to one of the most stable natural dyes: indigo, a deep blue dye. Indigo originates from plants like Indigofera tinctoria in India. From the 16th and 17th centuries, indigo was imported from India to Europe; in the 19th century, worldwide consumption rose sharply.
In 1868, Adolf von Baeyer determined the structural formula of indigo. Determining the structural formula is the most important prerequisite for the industrial synthesis of an organic compound. As described above, Adolf von Baeyer synthesized indigo chemically, leading to mass production by BASF in 1897. This breakthrough significantly reduced reliance on natural indigo and transformed textile industries worldwide.
In 1905, Baeyer was awarded the Nobel Prize in Chemistry in recognition of his contributions to the chemical industry and to research into organic dyes and aromatic compounds [1,8].
References/Sources
[1] Helmut Schmidt, Indigo – 100 Jahre industrielle Synthese, Chem. Unserer Zeit 2004. https://doi.org/10.1002/ciuz.19970310304
[1a] Adolf Baeyer, Synthese des Oxindols, Ber. Dtsch. Chem. Ges. 1878, 11(1), 582–584. https://doi.org/10.1002/cber.187801101153
[1b] Adolf Baeyer, Synthese des Isatins und des Indigblaus, Ber. Dtsch. Chem. Ges. 1878, 11(1), 1228–1229. https://doi.org/10.1002/cber.187801101337
[2] A. Baeyer, Ueber die Reduction des Indigblaus, Ber. Dtsch. Chem. Ges. 1868, 1(1), 17–18. https://doi.org/10.1002/cber.18680010107
[3] A. Baeyer, A. Emmerling, Reduction des Isatins zu Indigoblau, Ber. Dtsch. Chem. Ges. 1870, 3(1), 514–517. https://doi.org/10.1002/cber.187000301169
[4] A. Baeyer, A. Emmerling, Sur la réduction de l’isatine en indigo bleu, Bull. Soc. Chim. Paris 1870, 14(2), 416–417.
[5] A. Baeyer, Synthese des Indigblaus, Ber. Dtsch. Chem. Ges. 1878, 11(2), 1296–1297. https://doi.org/10.1002/cber.18780110206
[5] Adolf Baeyer, Ueber die Beziehungen der Zimmtsäure zu der Indigogruppe, Ber. Dtsch. Chem. Ges. 1880, 13(2), 2254–2263. https://doi.org/10.1002/cber.188001302243
[6] Adolf Baeyer, Viggo Drewsen, Darstellung von Indigblau aus Orthonitrobenzaldehyd, Ber. Dtsch. Chem. Ges. 1882, 15, 2856–2864. https://doi.org/10.1002/cber.188201502274
[7] K. C. Nicolaou, Tamsyn Montagnon, Molecules that Changed the World, Wiley-VCH, Weinheim, Germany, 2008. ISBN-13: 978-3527309832
[8] Roshan Paul, Richard S. Blackburn, Thomas Bechtold, Indigo and Indigo Colorants, in Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2021. https://doi.org/10.1002/14356007.a14_149.pub3
[9] Adolf Baeyer, Zur Geschichte der Indigo-Synthese, Ber. Dtsch. Chem. Ges. 1900, 33(3), LI-LXX. https://doi.org/10.1002/cber.190003303211