Peptides are important, e.g., in biological research and drug development. Methods for peptide synthesis are interesting research targets in chemistry. The activation of carboxylic acids using coupling reagents for the formation of amide bonds is a commonly used approach, for example, in the context of solid-phase peptide synthesis (SPPS). Useful coupling reagents need to be efficient and cost-effective, as well as retain the chiral information of the amino acid substrates.
Fei Lu, Peking University Shenzhen Graduate School, Shenzhen, China, Feng Yin, Pingshan Translational Medicine Center, Shenzhen, China, Zigang Li, Peking University Shenzhen Graduate School and Pingshan Translational Medicine Center, and colleagues have investigated the efficiency of different halopyridinium ions for SPPS. The team optimized the halopyridinium species by changing the halogen (Cl, Br, or I) and its position (2- or 4-halopyridinium).
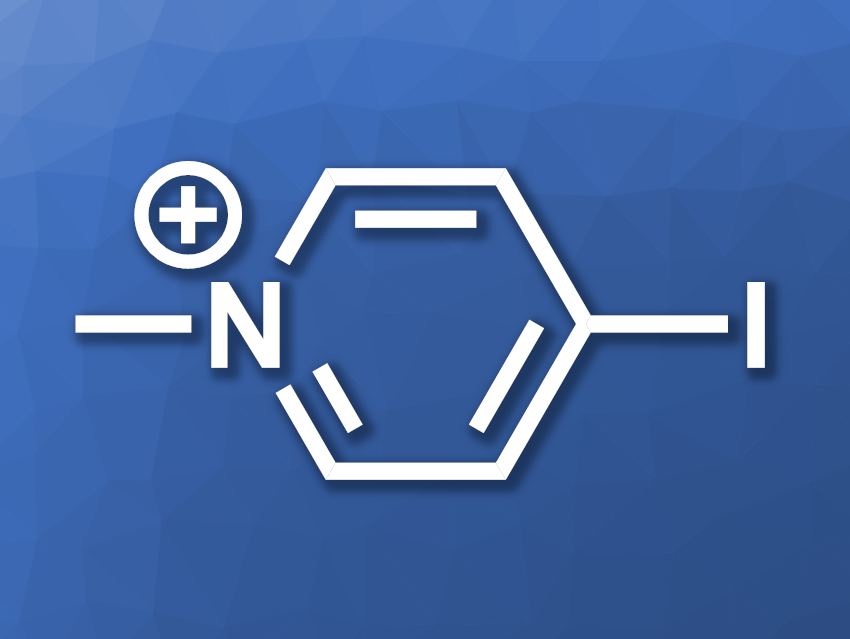
The researchers found that 4-iodine N-methylpyridinium (4IMP, pictured) is an efficient pyridinium-based coupling reagent. In the synthesis of a model peptide, using commercially available Fmoc-protected amino acids and 4-methylbenzhydrylamine (MBHA) resin, 4IMP provided the desired product with a purity of 96 % when combined with N-methylmorpholine (NMM) as the base and N,N-dimethylformamide (DMF) as the solvent. 4IMP can be easily prepared from inexpensive substrates, is bench-stable for extended periods, and could serve as an effective solid-phase peptide coupling reagent.
- 4-Iodine N-Methylpyridinium-Mediated Peptide Synthesis,
Wanjin Zhong, Chuan Wan, Ziyuan Zhou, Chuan Dai, Yichi Zhang, Fei Lu, Feng Yin, Zigang Li,
Org. Lett. 2023, 25, 8661–8665.
https://doi.org/10.1021/acs.orglett.3c03539