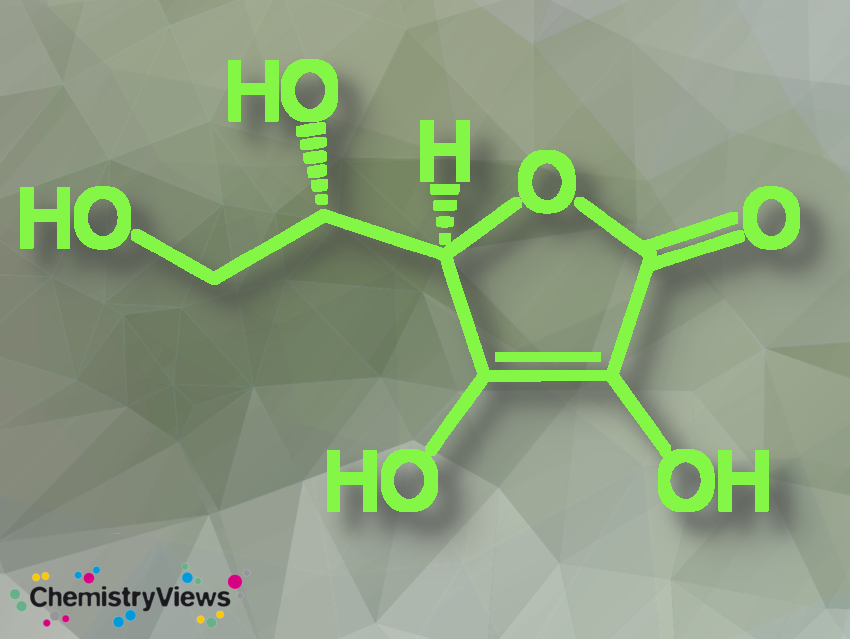
Vitamin C, L-ascorbic acid, ascorbic acid
Who?
probably independently by
- Tadeusz Reichstein (1897–1996; pictured)
- Walter N. Haworth (1883–1950) and Edmund Hirst (1898–1975)
Haworth and Hirst synthesized vitamin C on the laborator scale, Reichstein developed the first synthetic method to produce it in large-scale industrial production [1–5]. Haworth and Hirth say in their 1933 paper [2]: “It is now evident that to them [Reichstein, Grüssner, Oppenauer] belongs the credit of having published the first account of a synthetic derivate of the D-enantiomorph of ascorbic acid.”
When?
1933
How?
Through the Reichstein synthesis which was suitable for industrial production [1,3,4].
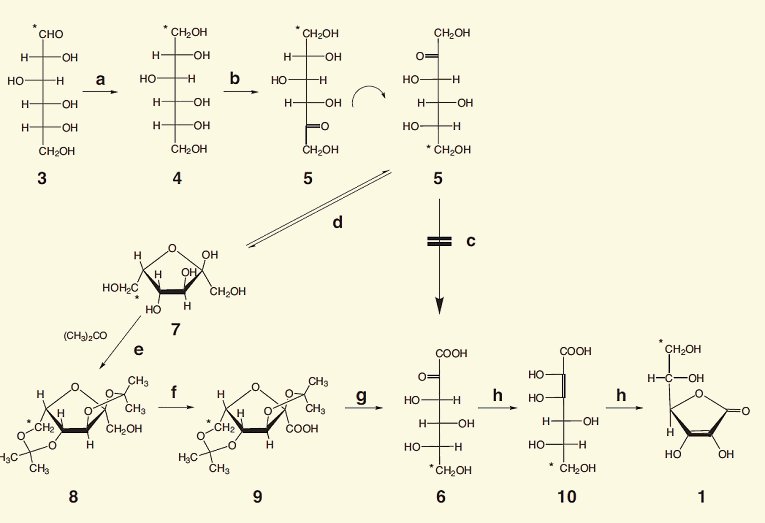
First, D-glucose (3) is hydrogenated to D-sorbitol (4) using nickel as a catalyst under high temperature and high pressure. Next, sorbitol is oxidized to L-sorbose (5) using Acetobacter xylinum or Gluconobacter oxydans at pH 4–6 and 30 °C.
Unfortunately, it is not possible to selectively oxidize the terminal, primary alcohol group in L-sorbose (5) into a carboxylic acid, because, regardless of which oxidizing agent is chosen, the other hydroxy groups are oxidized as well. For that reason, four of the five hydroxy groups are first protected against oxidation, all in a single step. As is true of most hexoses, L-sorbose exists in aqueous solution as an equilibrium mixture between the open-chain form (5) and a cyclic hemiacetal (7).
Reichstein discovered an ideal, economic, and environmentally friendly protective group for this purpose: acetone. The keto group of acetone can react with two adjacent hydroxy groups to give a cyclic acetal, and with a skillful choice of the reaction conditions, two molecules of acetone can be introduced to protect all four of the hydroxy groups in question against oxidation. This results in diacetone-L-sorbose (2,3:4,6−diisopropylidene−α−L−sorbose) (8). This is then oxidized to the acid 9.
In the presence of acid, acetone is cleaved off from the double acetal. In other words, both protective groups can be removed at once to yield 2-keto-L-gulonic acid (6). Finally, there is an acid-catalyzed tautomerization of 6 by way of 10 to the desired L-ascorbic acid (1), shown here in its cyclic form.
The microbial oxidation of sorbitol to sorbose is important because it provides the correct stereochemistry, since only the L-enantiomer is biologically active.
Very Short History of Vitamin C
Vitamin C (L-(+)-ascorbic acid) was first discovered 1907 by Axel Holst (1860–1931) and isolated by Albert Szent-Györgyi (1893–1986) and independently by Joseh L. Svirbely between 1828 and 1834. Initially it was named “hexuronic acid” [6].
Sir Walter Norman Haworth (1883–1950) identified its chemical structure as ascorbic acid, confirming its role as vitamin C. He and Szent-Györgyi introduced the name ascorbic acid [6]; the term comes from the Latin a– (meaning “not” or “without”) and scorbutus (Latin for scurvy).
Reichstein, along with his Ph.D. student Andreas Grüssner (1910–1999), as well as Haworth and his colleague Edmund Hirst synthesized vitamin C and published their synthesis in 1933 [1,2]. The Reichstein process was optimized, sold to the Swiss company Hoffmann-La Roche, and patented in 1933 [7,8], leading to the first commercially manufactured and sold vitamin product, Redoxon, in 1934 [9]. Today, the brand is owned by Bayer AG, Leverkusen, Germany.
Where?
Institut für Allgemeine und Analytische Chemie Eidg. Technische Hochschule (ETH Zurich) probably in the old chemistry building, Universitätsstr. 6, Zurich, Switzerland.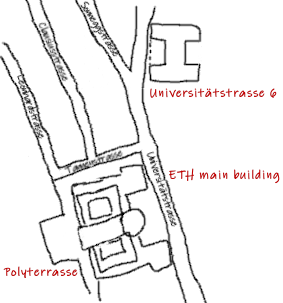
Original Building?
Yes
Architect?
The building was constructed between 1884 and 1886 by the architects Alfred Friedrich Bluntschli (1842–1830) and Georg Lasius (1835–1928) according to a program of two chemistry professors, Victor Meyer (1848–1897) and Georg Lunge (1839–1923) [10].
Brief History of the Person and the House
The chemistry building has housed many great chemists, including Richard Willstätter (1872–1943), Hermann Staudinger (1881–1965), Leopold Ružička (1887–1976), Vladimir Prelog (1906–1998), and Richard Ernst (1933–2021). Many of them were awarded the Nobel Prize for their research conducted in this building, contributing to the outstanding reputation of chemistry at ETH Zurich today.
The building was extended several times but eventually reached its maximum capacity and became too small to accommodate the rapidly growing field of chemistry. In November 2001, a modern chemistry building opened on the Science City campus on the Hönggerberg in a nice park [10] in Zurich.
Tadeusz Reichstein studied chemistry at ETH Zurich and earned his Ph.D. in 1921 under Hermann Staudinger with a dissertation on “Open-chain tropine and some of its homologues”. In 1929, he completed his habilitation with a thesis on the composition of the aroma compounds of roasted chicory and studies in the heterocyclic series within organic chemistry.
In 1931, he became an assistant to Leopold Ružička, and in 1937, he was appointed Associate Professor of Special Organic and Physiological Chemistry at ETH Zurich. From 1938, he headed the Pharmaceutical Institute at the University of Basel, Switzerland, and in 1946, he also assumed the Chair of Organic Chemistry. From 1960 to 1967, he served as Director of the Institute of Organic Chemistry at the University of Basel.
What Is It Today?
Today the building houses the ETH Department of Computer Science.
What is Tadeusz Reichstein Known For?
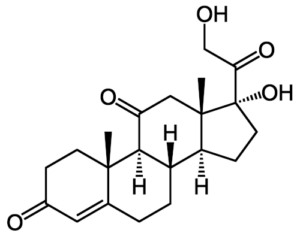 Reichstein also worked on hormones, leading to the isolation of cortisone, a steroid hormone (pictured on the right). Cortisone is an inactive compound, or prodrug, that is converted into its active form, cortisol, by the enzyme 11β-hydroxysteroid dehydrogenase type 1. In medications, cortisol is known as hydrocortisone. and can be used to treat conditions such as rheumatoid arthritis and asthma.
Reichstein also worked on hormones, leading to the isolation of cortisone, a steroid hormone (pictured on the right). Cortisone is an inactive compound, or prodrug, that is converted into its active form, cortisol, by the enzyme 11β-hydroxysteroid dehydrogenase type 1. In medications, cortisol is known as hydrocortisone. and can be used to treat conditions such as rheumatoid arthritis and asthma.
Reichstein was awarded the Nobel Prize in Physiology or Medicine in 1950 together with Edward Calvin Kendall (1886–1972) and Philip Showalter Hench (1896–1965) “for their discoveries relating to the hormones of the adrenal cortex, their structure and biological effects.”
References/Sources
[1] T. Reichstein, A. Grüssner, R. Oppenauer, Die Synthese der d-Ascorbinsäure (d-Form des C-Vitamins), Helv. Chim. Acta 1933, 16(1), 561–565. https://doi.org/10.1002/hlca.19330160177
[2] R. W. Herbert, E. L. Hirst, Synthesis of ascorbic acid, J. Soc. Chem. Ind. 1933, 52(31), 645-646. https://doi.org/10.1002/jctb.5000523107
[3] T. Reichstein, A. Grüssner, Eine ergiebige Synthese der L-Ascorbinsäure (C-Vitamin), Helv. Chim. Acta 1934, 17, 311–328. https://doi.org/10.1002/hlca.19340170136
[4] Klaus Roth, Vitamin C Deficiency – Part 2, ChemistryViews 2014. https://doi.org/10.1002/chemv.201400005
[5] W. N. Haworth, A. Szent-Györgyi, “Hexuronic Acid” (Ascorbic Acid) as the Antiscorbutic Factor, Nature 1933, 131, 24. https://doi.org/10.1038/131024b0
[6] K. C. Nicolaou, Tamsyn Montagnon, Molecules that Changed the World, Wiley-VCH, Weinheim, Germany, 2008. ISBN-13: 978-3527309832
[7] F Hoffmann La Roche AG, Verfahren zur Darstellung von 1-Ascorbinsäure (C-Vitamin), Patent CH187933, Switzerland, filed by Reichstein 1933.
[8] F Hoffmann La Roche AG, Process for the manufacture of 2-keto-levo-gulonic acid and product thereof, Patent US2039929A, United States 1935.
[9] REDOXON, SYNTHETIC CRYSTALLINE VITAMIN C, Classification Information, Trademarkia (accessed November 29, 2024)
[10] C. Goedecke, V. Koester, 24 Must-See Destinations in Europe for Chemists, ChemistryViews 2022. https://doi.org/10.1002/chemv.202200102
[11] Klaus Roth, The Biochemistry of Peppers – Part 2,