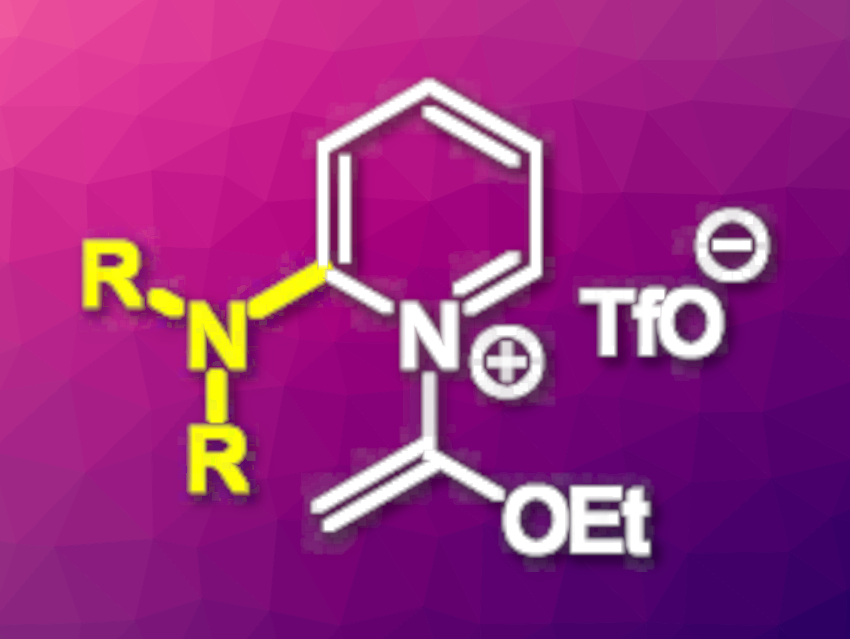
2-Aminopyridines are useful building blocks in organic chemistry, e.g., for drug development. Their synthesis can start from, for example, 2-halopyridines, which can undergo nucleophilic aromatic substitution (SNAr) reactions with amines. However, this type of transformation can require harsh reaction conditions or excess reagents, which also makes the purification of the products more difficult.
Max M. Majireck, Hamilton College, Clinton, NY, USA, and colleagues have found that bench-stable N-(1-ethoxyvinyl)-2-halopyridinium salts can undergo SNAr reactions with amine nucleophiles under unusually mild conditions to give 2-aminopyridines. This approach allows the reactions to take place under mild conditions and ambient atmosphere, while avoiding the use of transition metals, which can interfere in the drug discovery process. The team reacted a variety of 2-chloropyridinium triflate reagents with a range of primary and secondary amines, using K2CO3 as a base and CH2Cl2 as the solvent at 40 °C.
The resulting substitution products were obtained in moderate to excellent yields. The products, which contain the desired 2-aminopyridine subunit as well as pyridinium functionality, can be further transformed to remove the N-(1-ethoxyvinyl) substituent. This can be achieved by hydrolysis under acidic conditions or heating the product to 125 °C in isopropanol. Treating the products with an NaOH or NaHCO3 solution can give the free base, and an oxidation step can remove the N-(1-ethoxyvinyl) substituent and give a pyridine N-oxide at the same time. Overall, the work provides new reagents for the mild synthesis of 2-aminopyridines and 2-aminopyridinium salts.
- Bench-Stable 2-Halopyridinium Ketene Hemiaminals as Reagents for the Synthesis of 2-Aminopyridine Derivatives,
Isabelle C. Bote, Zoe A. Krevlin, Maria Christina F. Crespo, Sudchananya Udomphan, Carolyn T. Levin, Christie C. Lam, Amy M. Glanzer, Holly L. Hutchinson, Alisha M. Blades, Danielle L. McConnell, Crystal Lin, John P. Frank, William R. Strutton, Jordan C. Merklin, Beau A. Sinardo, Khady J. Gueye, Karly V. Leiman, Ashley Thayaparan, Joel K. A. Adade, Nestor L. Martinez, Wesley W. Kramer, Max M. Majireck,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c02915