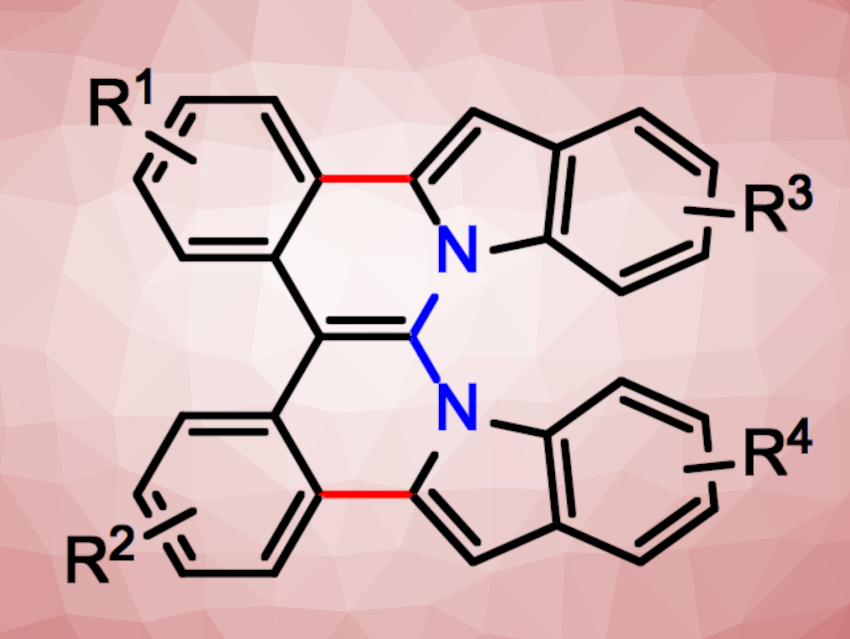
Helicenes are polycyclic aromatic hydrocarbons that form chiral helical shapes. These compounds can have interesting structural and optical features. Their properties can be tuned, for example, by introducing heteroatoms. In azahelicenes, one or more carbon atoms are replaced by nitrogen atoms, which can lead to considerable changes in the compound’s properties without large structural changes. 1,8-Naphthyridines are naphthalene derivatives with one nitrogen atom in each ring. So far, there had been no examples of double azahelicenes with a 1,8-naphthyridine core.
Peter Ehlers, University of Rostock, Germany, and colleagues have synthesized such double azahelicenes (general structure pictured). The team started from 1,2-dibromobenzenes, which were reacted with methyl 2,2-difluoroacetate to obtain CHF2-substituted tertiary carbinols. These intermediates were dehydrated to give 2,2-di(2-bromophenyl)-1,1-difluoroalkenes.
The difluoroalkenes were reacted with indole derivatives in a nucleophilic fluorine substitution to obtain precursors for the desired aza[4,6]helicenes. These X-shaped azahelicenes were obtained from these precursors via a palladium-catalyzed ring-closing reaction in yields up to 60 %. The nucleophilic fluorine substitution and the ring-closing step were performed in a one-pot process. The approach allows for different substitution patterns in the azahelicene products.
- Dibenzoindolo[1,8]naphthyridines: Synthesis and Characterization of X‐Shaped Aza[4,6]helicenes,
Elina Ausekle, Peter Ehlers, Alexander Villinger, Peter Langer,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202303225