1,3-Oxazole derivatives are useful in medicinal chemistry due to their ability to interact with various molecular targets in cells, as well as their structural diversity. They can serve as promising candidates in the discovery of new therapeutic agents.
Yevheniia Velihina, V. P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry, National Academy of Sciences of Ukraine, Kyiv, and INSA Rouen Normandie, Mont-Saint-Aignan, France, Volodymyr Brovarets, V. P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry, and colleagues have developed a series of 5-sulfinyl(sulfonyl)-4-arylsulfonyl-substituted 1,3-oxazoles with anticancer properties.
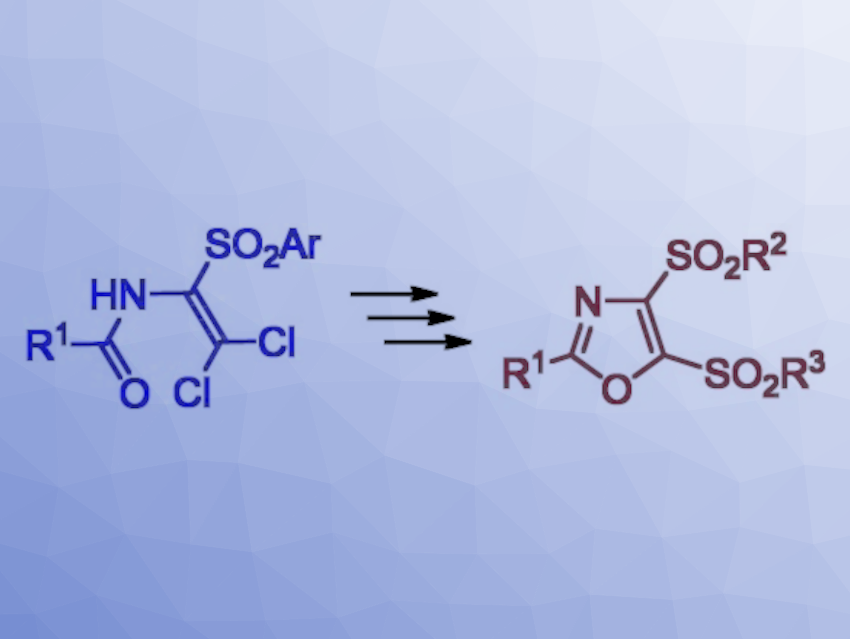
The team used N-(2,2-dichloro-1-(arylsulfonyl)vinyl)amides as starting materials (pictured above on the left). They were reacted with sodium sulfide and alkyl halides to form 4-arylsulfonyl-5-alkylthiooxazoles, which were then oxidized to obtain 5-sulfinyl derivatives and 5-sulfonyl derivatives. Alternatively, the starting materials were reacted with arenethiols, followed by cyclization to form 4,5-bis(arylthio)oxazoles. The corresponding sulfonyl derivatives were, again, obtained by oxidation.
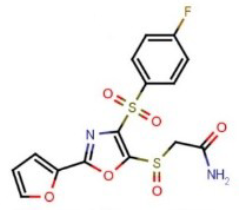
The anticancer properties of the resulting oxazoles were investigated using a panel of 60 different human cancer cell lines derived from lung, colon, skin, kidney, ovarian, brain, blood, breast, and prostate cancers. The team found that cancer cell lines of all subpanels were most sensitive to 2-{[4-[(4-fluorophenyl)sulfonyl]-2-(2-furyl)-1,3-oxazol-5-yl]sulfinyl}acetamide (pictured below). This compound could, thus, serve as a lead compound for further studies and the synthesis of new 1,3-oxazole derivatives with anticancer activity.
- Synthesis, Characterization and in vitro Anticancer Evaluation of 5‐Sulfinyl(sulfonyl)‐4‐arylsulfonyl‐Substituted 1,3‐Oxazoles,
Vladimir Zyabrev, Stepan Pilyo, Bohdan Demydchuk, Maryna Kachaeva, Ivan Semenyuta, Victor Zhirnov, Yevheniia Velihina, Volodymyr Brovarets,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202300161