Organoboranes are useful intermediates for the synthesis of pharmaceuticals and fine chemicals. One method for the formation of organoboranes is the direct carboboration of a double- or triple bond, simultaneously forming a new C−C and C−B bond. However, such carboboration reactions are often limited to specific Lewis-acidic boranes.
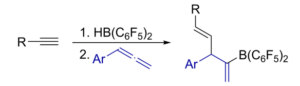
Urs Gellrich, University of Gießen, Germany, and colleagues have developed a more general approach to synthesizing Lewis-acidic alkenylboranes, which can then readily undergo carboboration reactions (overall reaction pictured below). The team used a simple hydroboration of alkynes with HB(C6F5)2 to obtain a variety of alkenylboranes in situ. These boranes were then directly reacted with arylallenes (pictured in blue below) in a regioselective 1,2-carboboration under mild conditions.
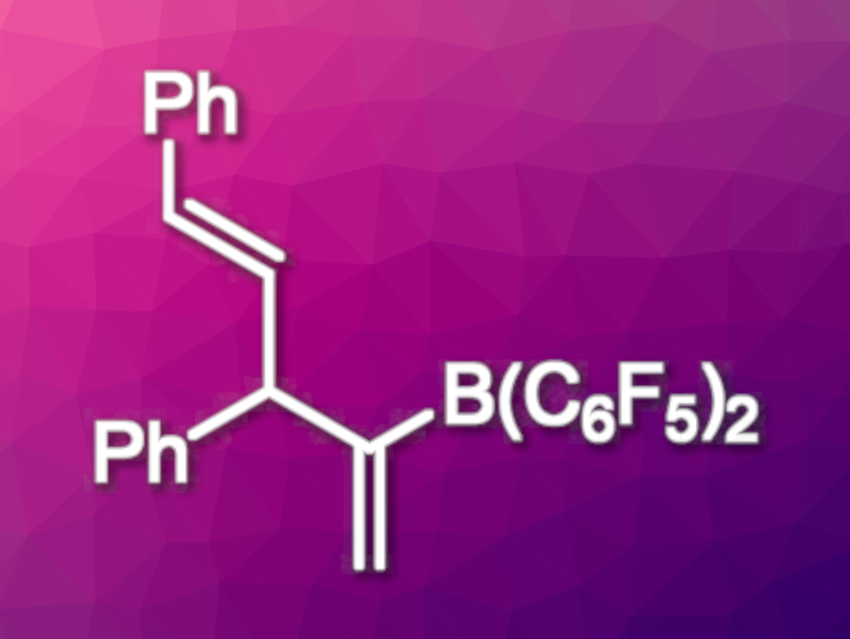
The products of this reaction protocol are well suited for further transformations, e.g., for palladium-catalyzed cross-couplings with aryl iodides. The generality of the resulting three-step, one-pot protocol was demonstrated by the synthesis of 20 different aryl-substituted 1,4-dienes. This protocol is a promising method for the modular coupling of alkynes, allenes, and aryl iodides.
- 1,2‐Carboboration of Arylallenes by In Situ Generated Alkenylboranes for the Synthesis of 1,4‐Dienes,
Arthur Averdunk, Max Hasenbeck, Tizian Müller, Jonathan Becker, Urs Gellrich,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202200470