François Antoine Philippe Barbier was born on March 2, 1848, in Luzy, France. Little is known about his early life until he started his university education. Barbier worked under Marcelin Berthelot at the Collège de France, Paris, starting in 1869, and received his Ph.D. in 1876. He then served as a Laboratory Assistant at the École Supérieure de Pharmacie, Paris, and became Associate Professor at the Unversity of Lyon in 1878.
From 1879 to 1884, Barbier worked at the University of Besançon, France, first as Lecturer and then as Professor. He then returned to Lyon as Professor of Chemistry and remained there for the rest of his career. Philippe Barbier died on September 18, 1922, in Bandol-sur-Mer, France.
The Barbier and Grignard Reactions
Philippe Barbier is often considered the “father” of organometallic chemistry. He is well known for the creation of organomagnesium reagents with his doctoral student Victor Grignard, the Barbier reaction, and the Barbier-Wieland degradation. He also worked on terpene chemistry and mineralogy.
Barbier synthesized the first organomagnesium nucleophiles in 1899. He developed a reaction of methyl iodide with a methylheptenone in the presence of magnesium to give a dimethylheptenol [1]—an example of a Barbier reaction (pictured below). In general, a Barbier reaction is a reaction between an alkyl halide, a carbonyl reagent, and a metal such as magnesium, lithium, aluminium, zinc, indium, tin, or samarium.
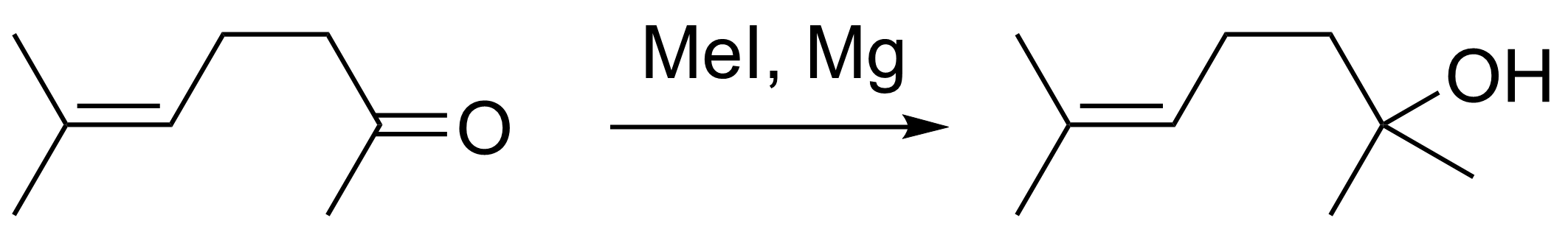
However, this new reaction had some issues with yields and reproducibility. Victor Grignard was tasked with optimizing the reaction conditions. In 1900, Grignard published an improved procedure in which the organomagnesium compound is formed first, followed by addition of the carbonyl substrate [2].
The Nobel Prize in Chemistry 1912 was awarded to Grignard “for the discovery of the so-called Grignard reagent, which in recent years has greatly advanced the progress of organic chemistry” and Paul Sabatier for his work on the hydrogenation of organic compounds in the presence of metals. The omission of both Barbier and Sabatier’s collaborator Jean-Baptiste Senderens was controversial. Grignard stated that he would have preferred to share his part of the prize with Barbier [3]. Barbier destroyed many records of his life and career after the 1912 Nobel Prize, and there has been some speculation that the two events are linked.
The Barbier–Wieland Degradation
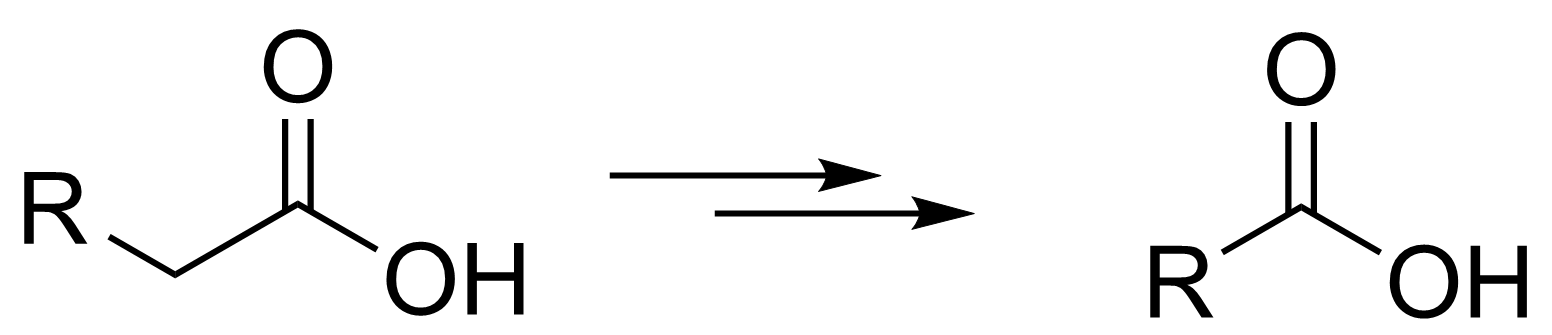
The Barbier–Wieland degradation can be used to shorten the carbon chain of a carboxylic acid by one C atom (pictured below). It was developed by Barbier [4] and Heinrich Wieland [5]. The acid is first converted to an ester and then subjected to two consecutive Grignard reactions to give an alcohol. The alcohol is transformed to an alkene using an elimination, and the alkene is oxidized and cleaved to form the desired shortened acid.
Philippe Barbier is the Answer to Guess the Chemist (129).
References
[1] P. Barbier, Synthèse du diéthylhepténol, Compt. Rend. 1899, 128, 110.
[2] V. Grignard, Sur quelques nouvelles combinaisons organometalliques du magnesium et leur application a des syntheses d’alcools et d’hydrocarbures, Compt. Rend. 1900, 130, 1322.
[3] T. Urbański, Chem. Brit. 1976, 12, 191–192.
[4] P. Barbier, R. Locquin, Dégradation méthodique de divers acides saturés mono et bibasiques, Compt. Rend. 1913, 156, 1443.
[5] H. Wieland, Über Hydrierung und Dehydrierung, Ber. dtsch. chem. Ges. 1912, 45, 484–493. https://doi.org/10.1002/cber.19120450171
Sources
- D. Lewis, Philippe Barbier (1848–1922) and Victor Grignard (1871–1935): Pioneers of Organomagnesium Chemistry, Synform 2018, 10, A155. https://doi.org/10.1055/s-0037-1609793
- V. V. Mainz, G. S. Girolami, Genealogy database entry: P. Barbier, based on Bull. Soc. Chim. Fr. 1922, 31, 1244–1245.
- H. Rheinholdt, Fifty years of the Grignard reaction, J. Chem. Educ. 1950, 27, 476. https://doi.org/10.1021/ed027p476



