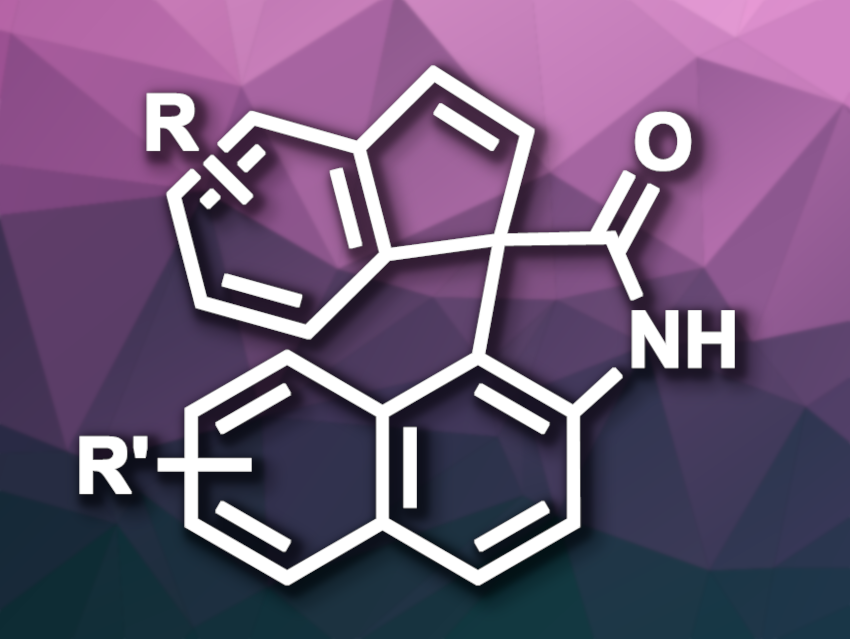
1,1′-Bi-2-naphthol (BINOL) derivatives are widely used, e.g, as ligands in asymmetric synthesis. 2′-amino-[1,1′-binaphthalen]-2-ol (NOBIN) is an amino-functionalized BINOL derivative that can also be used as a ligand, but could also serve as a precursor for useful fused heterocyclic compounds. However, its chemistry has been less well-explored than that of BINOL so far.
Frederic W. Patureau, RWTH Aachen University, Germany, and colleagues have a developed a method for the oxidation of NOBIN derivatives that gives α-spiropyrrolidones, products with an interesting heterocyclic framework. The team reacted a variety of substituted NOBINs with oxidants to induce a skeletal rearrangement. They found that the hypervalent iodine compound phenyliodine(III) diacetate (PIDA) is a suitable oxidant, together with H3CONa as a base. The reactions were performed in chloroform at room temperature.
Under these conditions, the desired α-spiropyrrolidones were obtained in mostly moderate to good yields. The reactions tolerates a range of substituents in different positions of the substrate, however, an N-phenyl substituent led to a very low yield, and a substrate with a 3-carboxyl ester group was entirely unsuitable. According to the researchers, the work could be useful for researchers developing oxidative methods in organic synthesis, as well as for the preparation of heterocyclic systems.
- Oxidation of NOBINs Toward α-Spiropyrrolidones,
Yun Yang, Ben Ebel, Iris M. Oppel, Frederic W. Patureau,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c02499