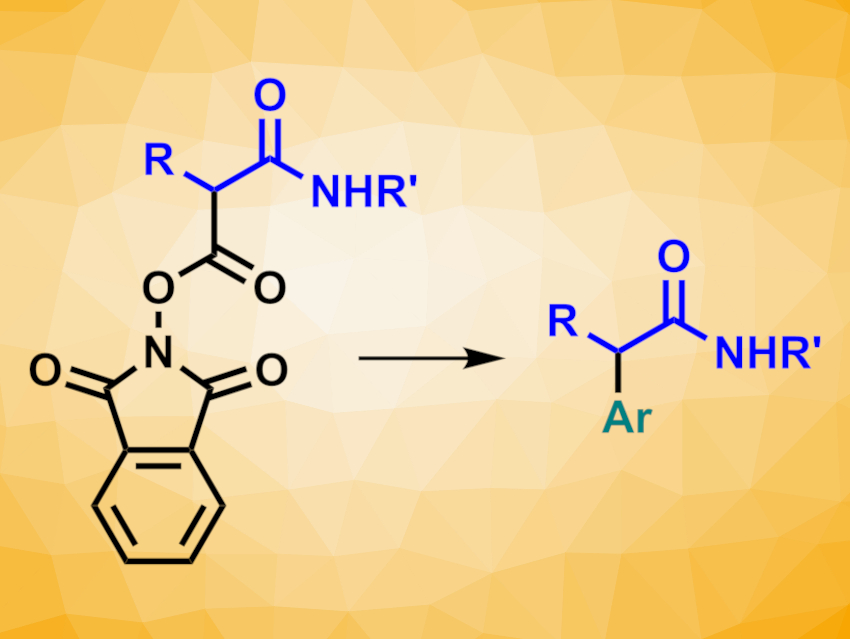
α-Aryl amides are commonly found, e.g., in biologically active compounds and other complex organic molecules. Thus, the transition-metal-catalyzed α-arylation of amides can be a useful transformation in organic synthesis. There is a range of methods for the α-arylation of tertiary amides. The α-arylation of secondary amides is more challenging due to their free N–H bond, which can be deprotonated under the basic conditions often required for the arylation reactions.
Sophie A. L. Rousseaux, University of Toronto, Canada, and colleagues have developed a method for the nickel-catalyzed synthesis of secondary α-aryl amides under mild conditions. The team used a reductive cross-coupling with redox-active N-hydroxyphthalimide (NHP) esters of malonic acid half amides (pictured). These esters are readily prepared from Meldrum’s acid (2,2-dimethyl-1,3-dioxane-4,6-dione).
The team reacted a range of these NHP esters with different substituted aryl iodides. They used NiCl2(bpy) as a catalyst (bpy = 2,2′-bipyridine), PhMeSiCl2 as an additive, Mn and Zn as reductants, and dimethylacetamide (DMA) as the solvent. The reactions were performed at 0 °C.
The desired arylated products were obtained in moderate to good yields. The reaction has a broad scope and tolerates other functional groups that might undergo cross-coupling reactions, e.g., aryl chlorides and aryl bromides, as well as base-sensitive groups.
- Synthesis of α-Aryl Secondary Amides via Nickel-Catalyzed Reductive Coupling of Redox-Active Esters,
Alexis L. Gabbey, Nicholas W. M. Michel, Jonathan M. E. Hughes, Louis-Charles Campeau, Sophie A. L. Rousseaux,
Organic Letters 2022.
https://doi.org/10.1021/acs.orglett.2c00918