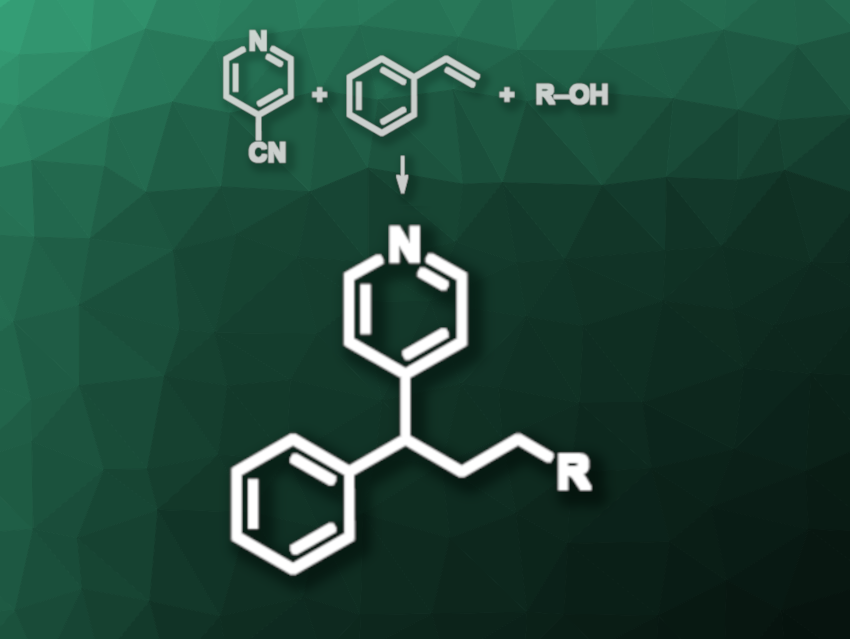
Pyridine derivatives are often used, e.g., in pharmaceutical chemistry. Metal-free, modular paths to pyridine derivatives could be particularly interesting in drug development. Radical multicomponent reactions can be used to efficiently synthesize complex molecules from more than two substrates. Deoxygenative variants of this type of reaction that form two consecutive C–C bonds are, however, rare.
Pei-Xin Rui, Xiang-Guo Hu, Jiangxi Normal University, Nanchang, China, and colleagues have developed a metal-free, deoxygenative three-component reaction involving alcohols, styrenes, and cyanopyridines that gives substituted pyridine derivatives (example reaction pictured). The team used an N-heterocyclic carbene (NHC) to activate the alcohol in methyl tert-butyl ether (MTBE) in the presence of pyridine as a base. Then they employed a tetrakis(dibromo-9H-carbazolyl)phthalonitrile as a photocatalyst, tetramethylguanidine (TMG) as a base, acetonitrile as an additional solvent, and blue LED irradiation to promote the reaction.
Under these conditions, the alcohol is activated, oxidized by the excited-state photocatalyst in a single-electron transfer (SET), deprotonated, and deoxygenated to give an alkyl radical. The alkyl radical can add to the styrene, giving a benzyl radical. The cyanopyridine is reduced by the photocatalyst to give a radical anion, which can couple with the benzyl radical and form the desired product under loss of CN–. Overall, the products were obtained in moderate to good yields, the reaction is compatible with a wide range of alcohols, and the method can be used for the late-stage modification of complex molecules.
- Radical Deoxygenative Three-Component Reaction of Alcohols, Aryl Alkenes, and Cyanopyridines,
Li-Min Feng, Shuai Liu, Yuan-Hong Tu, Pei-Xin Rui, Xiang-Guo Hu,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c02150