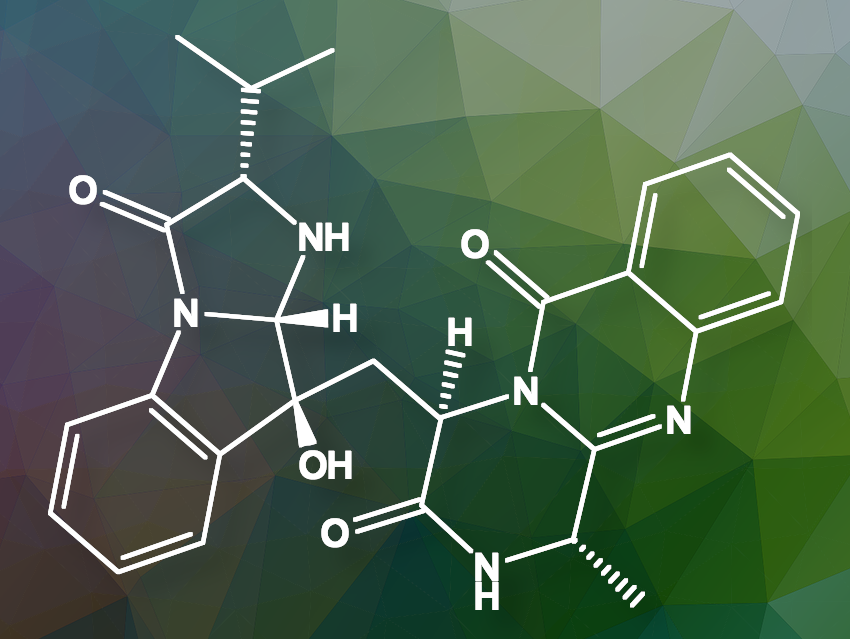
Roxana Damiescu and Thomas Efferth from the University of Mainz, Germany, and colleagues have found that aniquinazoline B (pictured above), a quinazolinone alkaloid derived from the marine fungus Aspergillus nidulans, binds to and activates an opioid receptor, providing pain relief. However, unlike traditional opioids, it does not cause addiction or respiratory depression.
How did you discover aniquinazolin B?
By using a bioinformatics-based approach and the supercomputer MOGON at the Johannes Gutenberg University Mainz, we have performed large-scale virtual drug screening. In an attempt to identify new agonists for the µ-opioid receptor, we screened a chemical library of 40,000 natural-product-based products.
The top 100 ligands from this virtual drug screening were then used to predict ligand position and binding affinity using molecular docking. The results of the first drug screening were then validated with a second in silico technique.
Afterward, the ten best candidate compounds from this computational approach were experimentally tested in a drug-binding assay (microscale thermophoresis) and a functional assay (cAMP assay). Microscale thermophoresis is a highly sensitive method for determining the binding affinity of a ligand to a protein in its native state. In contrast, the cAMP assay is a high-throughput method to measure the intracellular signal transducer cAMP upon treatment. Subsequently, we focused on aniquinazoline B, a compound from the marine fungus Aspergillus nidulans as the most promising candidate.
As a next step, we performed transcriptomic expression profiling to uncover the genes and signaling pathways that were deregulated upon treatment with aniquinazoline B. As an example, we validated the mRNA expression data by qRT-PCR and western blotting, and found that two G protein-coupled signaling pathways linked to the µ-receptor were activated by aniquinazoline B.
Why are you doing this?
In recent years, the misuse of analgetic drugs has caused a severe crisis, the so-called opioid crisis.
Opioids are the strongest analgesics that can be administered to manage severe acute and chronic pain. Because of their addictive potential, opioids have been frequently misused and cause thousands of overdose-related deaths. It has been estimated that more people die annually from opioid misuse than soldiers who died in the entire Vietnam War.
The opioid crisis has become a severe public health problem in many countries, including the USA, Canada, and the UK. Therefore, there is an urgent need to develop new drugs with a strong analgesic effect but no (or less) addictive potential. As natural products are a valuable and well-known source for new therapeutics, our aim was to use this asset to uncover new candidates for alternative pain treatment.
What is new and cool about your method?
Virtual drug screening represents a promising concept to identify novel drug candidates. We took advantage of the supercomputer MOGON at the Johannes Gutenberg University, which is one of the largest supercomputers in Germany and even worldwide.
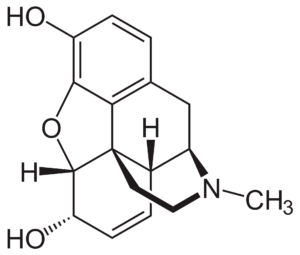 We find it very interesting that our drug candidate is a fungal natural product with a chemical structure completely different from that of opioids (e.g., morphine (pictured on the right), oxycodone).
We find it very interesting that our drug candidate is a fungal natural product with a chemical structure completely different from that of opioids (e.g., morphine (pictured on the right), oxycodone).
The quinazoline alkaloids have a very broad range of pharmacological activities due to their diverse structures, including antiparasitic and insecticidal, antibacterial and antifungal, cardioprotective, antiviral, anti-inflammatory, making them a fascinating chemical class.
Microscale thermophoresis provides a fast and flexible method to experimentally confirm the results obtained in silico. Additionally, through transcriptomic analysis, we managed to uncover valuable information regarding the pathways and networks affected by our compound downstream of the µ-opioid receptor.
What are your key findings?
We have successfully identified aniquinazoline B as a new compound that activates the µ-opioid receptor and managed to uncover many important aspects of its predicted pharmacodynamic properties, thus taking an important step forward in the mechanistic understanding of how this drug works.
How will you proceed now?
Considering the long time required for a drug to be developed from the bench to the bedside, we consider that the next important steps include animal testing, to see how the compound works in vivo, to understand the pharmacokinetics, and to perform the necessary toxicity testing. Secondly, we will develop further derivatives and investigate their safety and efficacy in inhibiting pain.
What part of your work was the most challenging?
The most challenging part was without doubt the virtual drug screening. Preparing and testing a library of 40,000 compounds in a way that testable candidates can be identified is not a trivial task. This task would not have been possible without the supercomputer MOGON.
Thank you for these insights.
The article they talked about
-
- Aniquinazoline B, a fungal natural product, activates the µ‐opioid receptor,
Roxana Damiescu, Mohamed Elbadawi, Mona Dawood, Sabine M. Klauck, Gerhard Bringmann, Thomas Efferth,
ChemMedChem 2024.
https://doi.org/10.1002/cmdc.202400213
- Aniquinazoline B, a fungal natural product, activates the µ‐opioid receptor,


Roxana Damiescu is a Ph.D. student in the Department of Pharmaceutical Biology at the Institute of Pharmaceutical and Biomedical Sciences of the Johannes Gutenberg University in Mainz, Germany.
Professor Thomas Efferth is Director of the Institute of Pharmaceutical and Biomedical Sciences and Chair of the Department of Pharmaceutical Biology at Johannes Gutenberg University. He serves as Editor-in-Chief of Phytomedicine and is an Ordinary Member of the Academia Europaea (London).