Electrocatalytic water splitting reaction could be an important tool in the transition to renewable energy technologies, since hydrogen produced using sustainable electricity could be useful as an environmentally friendly fuel. The development of suitable catalysts for the electrochemical hydrogen evolution (HER) is, thus, an important research topic. Catalysts used for this type of reaction are often based on precious metals such as platinum. Metal-free alternatives, i.e., organocatalysts, are interesting, but not very well explored. Existing examples have shown small current densities that are too low for practical use and do not work well in aqueous solutions.
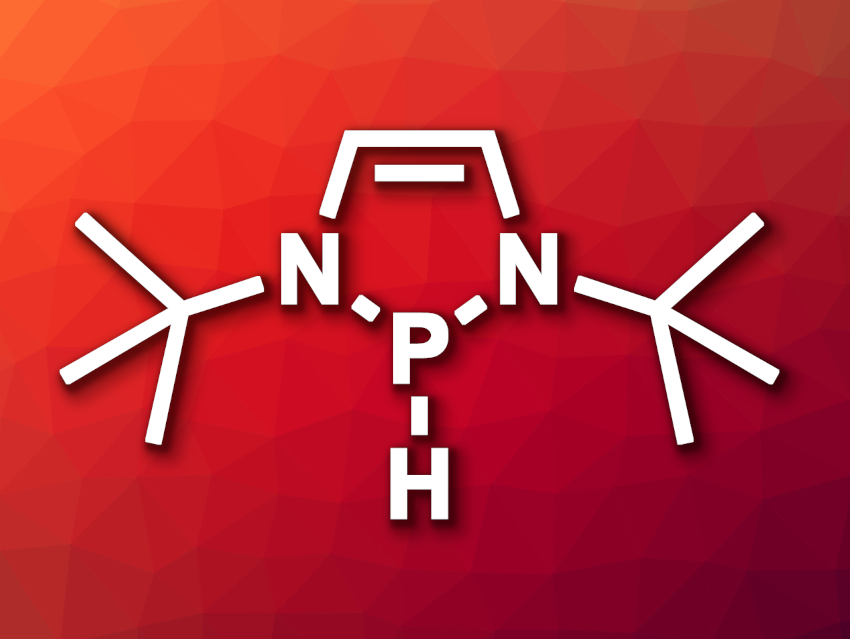
Long Zhang, Jin-Dong Yang, Jin-Dong Yang, Tsinghua University, Beijing, China, and colleagues have found that N-heterocyclic phosphines (NHPs, example pictured) are organohydrides that can serve as effective catalysts for the electrochemical hydrogen evolution. The optimized catalyst features two tert-butyl substituents. Its bromo-functionalized precursor, i.e., a P-bromogenodiazaphospholene, was treated with Nafion, a polytetrafluoroethylene (PTFE)-based polymer that contains sulfonic acid groups, to obtain a NHP-sulfonate solution. This solution was then deposited onto a carbon paper cathode, which was used for the HER.
According to the researchers, the developed HER organocatalyst showed the best activity achieved with a metal-free, small-molecule catalyst so far. The team proposes that the precatalyst is protonated by the sulfonic acid to induce dissociation of the P–Br bond and give a phosphenium species (NHP+). This cation can undergo a one-electron reduction to form a radical (NHP•), followed by protonation to give a radical cation (NHP-H•+), and a second one-electron reduction to give the hydride (NHP-H). This last species is a hydride donor, which reacts with a proton under the acidic conditions in the electrolyte and forms the desired H2. The researchers state that the catalyst may also have potential for other reductive processes.
- Organocatalytic Hydrogen Evolution Reaction by Diazaphospholenes,
Kaini Xu, Yu-Shan Zhang, Bing Zhong, Long Zhang, Jin-Dong Yang, Sanzhong Luo,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c10302