Peptides are the biological little brothers of proteins, consisting of 100 amino acids at the most. They exhibit a broad range of biological functions, for example, as hormones or natural antibiotics. Whereas certain proteins are often found to be bound to hydrophobic fatty acids or phospholipids in order to keep them close to the cell membrane, lipidation of peptides is unknown in nature. Nevertheless, synthetic phospholipopeptides might serve as effective therapeutics.
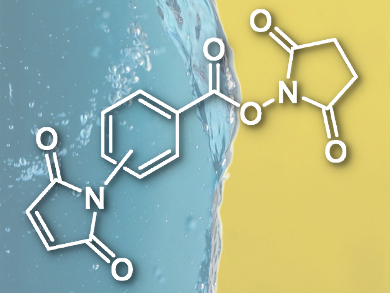
Bonan Li and Jun F. Liang, Stevens Institute of Technology, Hoboken, NJ, USA, report an approach to synthesize phospholipopeptides. They use a crosslinker with a thiol-reactive maleimide and an amine-reactive N-hydroxysuccinimide ester (pictured). Hence, the molecule is able to link the thiol group of the amino acid cystein in the peptide and the amine group of the phospholipid (phosphatidylamine).
The team is able to synthesize lipopeptides in high yield (over 90 %) under mild conditions, while avoiding the necessity of protecting various nucleophilic groups in the peptide. Neither the length of the peptide, nor the amino acids found next to the connecting cysteine have any negative impact on the reaction outcome.
- A linker approach to phospholipopeptides,
Bonan Li, Jun F. Liang,
Eur. J. Lipid Sci. Technol. 2015.
DOI: 10.1002/ejlt.201400593