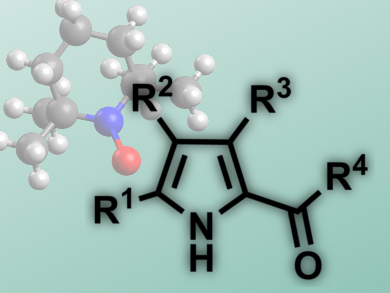
Radical processes usually feature mild reaction conditions and good functional group tolerance. However, they usually require hazardous reagents like organotin or azo compounds.
Steven L. Castle and colleagues, Brigham Young University, Provo, UT, USA, have reported a tin-free iminyl cyclization with alkyne acceptors that needs no radical initiatiors and leads to 2-acylpyrroles (pictured). The starting materials are O-phenyl oxime ethers, easily generated from the corresponding ketones, which feature a weak N–O bond. Microwave heating of the reaction mixture is sufficient to break this bond and initiate the reaction, a 5-exo-dig cyclization.
The researchers use TEMPO, a stable radical, to trap the radical intermediate. Subsequent isomerization and fragmentation leads to the products in good yields (53 – 98 %). The reaction tolerates a range of substituents at the pyrrole, as well as different acyl groups and cyclic substrates. This 2-acylpyrrole synthesis presents an alternative to Friedel-Crafts-Acylations or Vilsmeier reactions, featuring mild conditions, good yields, and non-hazardous reagents.
- Microwave-Promoted Tin-Free Iminyl Radical Cyclization with TEMPO Trapping: A Practical Synthesis of 2-Acylpyrroles,
Yu Cai, Ankur Jalan, Aaron R. Kubosumi, Steven L. Castle,
Org. Lett. 2015.
DOI: 10.1021/ol5035047